To Issue 172
Citation: “Interview with Laure-Hélène Guillemot: Understanding the Impact of USP<382> on Primary Packaging Development”. ONdrugDelivery, Issue 172 (May 2025), pp 14–15.
Laure-Hélène Guillemot discusses the new USP <382> chapter coming into force on December 1st, 2025, which will significantly transform functional testing protocols for pharmaceutical companies. This new standard mandates that elastomeric components, such as stoppers and seals, meet stringent safety and efficacy criteria, including tests for container closure integrity, needle and spike access functionality, and the performance of plungers and stoppers. Dr Guillemot explains the impact on pharmaceutical companies’ product development approaches and how Aptar Pharma can support its customers from a functional testing perspective.
Q What is the objective of USP <382> compared with USP <381>, and how does it impact pharmaceutical companies?
A USP <381> “Elastomeric Components in Injectable Pharma Products Packaging” refers to the evaluation of elastomeric compounds individually, not considering them as part of a full system. As such, the stopper is evaluated without taking the potential impact of its interactions with the vial into account, the crimping cap and the drug product on its functional performances.
This USP chapter contains three sections – biological reactivity, functional tests and physical-chemical tests. The biological reactivity and physical-chemical tests sections apply to all elastomeric compounds, such as stoppers for vials and plungers for PFSs, whereas the functional tests only apply to elastomeric compounds meant to be pierced, such as stoppers for vials.
The objective of USP <382> “Elastomeric Component Functional Suitability in Parenteral Product Packaging/Delivery Systems” is to evaluate the functionality of the complete system instead of the individual elastomeric compounds alone. The novelty is that it includes not only stoppers but also plungers and needle-shields, so systems for injectable applications, such as vials, PFSs and cartridges, are therefore included. Pharmaceutical companies, being the stakeholders owning the complete packaging information, will therefore be responsible for checking the full compliance of its systems towards USP <382>.
As of December 1st, 2025, USP <381> will remain the reference for biological reactivity and physical-chemical parts. Following the USP compendial notice published on April 25th, 2025, fragmentation testing will also remain applicable as described in USP <381>, while penetrability and self-sealing tests will be omitted from functionality tests of USP <381> to be included to USP <382> chapter (Figure 1).
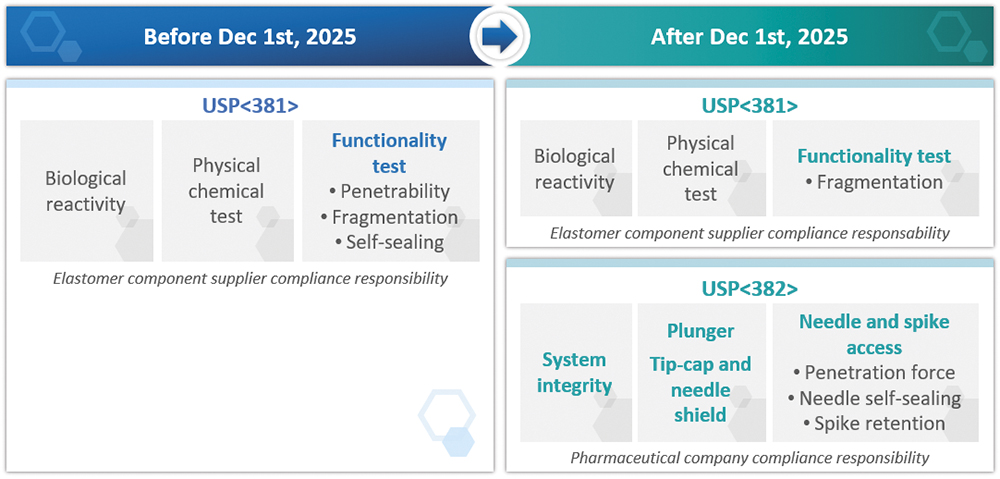
Figure 1: Implementation of USP <382> with regards to USP <381> and its impact for drug developers/pharmaceutical companies.
Q Which new tests are required with USP <382> and which modifications are made to existing tests?
A The main recommendation from USP <382> is to evaluate the full system according to its final intended use. Pharmaceutical companies are responsible for setting acceptance criteria and developing testing methods that best represent their systems’ real-life conditions of use. Although USP <382> does not impose specifications, it provides pharmaceutical companies with guidelines.
For infusion applications, the spike retention and sealability capacity test has been added as part of USP <382> to ensure that the spike does not slide out of the pierced rubber and that the solution does not leak at the stopper/spike interface. The penetration force and self-sealing capacity tests are not new, but have been reviewed to consider the product’s final intended use.
As an example, for vial systems containing dry products that need reconstitution, USP <382> recommends evaluating self-sealing capacity using 30 stoppers pierced one time each with an 18G needle, then at least three times with a 21G needle. In comparison, USP <381> did not consider the need for reconstitution with an 18G needle and self-sealing tests only required 10 stoppers to be pierced 10 times with 21G needles.
For syringe barrel-plunger systems, USP <382> requires break-loose, extrusion force (also known as gliding force) and plunger seal integrity tests to be performed. These requirements are considered “new” as they were not previously described in the pharmacopeia, but such tests were already routinely performed by PFS and component manufacturers. This is also the case for the pull-off force and torque force tests now required for systems using tip caps or needle shields.
For both vials and PFS systems, USP <382> strongly recommends performing deterministic container-closure integrity testing rather than using a probabilistic method.
Q How will the drug development process be affected and what should pharmaceutical companies do to comply with the new requirements of USP <382>?
A Pharmaceutical companies and drug developers are the only stakeholders owning all information related to their drug and delivery systems. Under USP <382>, it is their responsibility to ensure the compliance of their complete vial system (stopper, vial, crimping cap and drug or placebo) or PFS system (barrel, plunger, tip closure and drug or placebo).
“FOR PRODUCTS IN DEVELOPMENT PHASE, DRUG DEVELOPERS MUST INCLUDE AN EVALUATION ACCORDING TO USP <382> AND ALL DRUG PRODUCTS SUBMITTED AS OF DECEMBER 1ST, 2025, WILL HAVE TO COMPLY WITH THIS NEW REGULATION.”
For products already on the market, there is no obligation to carry out these tests. However, starting December 1st, 2025, following changes for existing products, pharmaceutical companies will have to assess whether product functionality is affected and may be required to provide additional technical data to demonstrate compliance with USP <382>. For products in development phase, drug developers must include an evaluation according to USP <382> and all drug products submitted as of December 1st, 2025, will have to comply with this new regulation.
Q How can Aptar Pharma support drug developers in complying with USP <382>?
A Aptar Pharma has already performed a series of tests on its vial stopper market standards according to USP <382> guidelines. Results and documentation are available upon request and can help drug developers in making informed decisions when selecting their drug delivery system. Furthermore, as test methods must now represent the final condition of use of the drug product, Aptar Pharma’s R&D specialists can work with drug developers to design situation specific tests and inform the choice of primary packaging.
Aptar Pharma has also performed a risk assessment and identified adjustments that need to be made to the quality documentation. For the latest stages of drug development and regulatory filing, drug developers may require assistance in functional testing to meet the requirements of USP <382>. Gateway Analytical, an Aptar Pharma company, offers GMP analytical and testing services.
In conclusion, Aptar Pharma’s teams have been working to understand and anticipate the changes that will come into effect with the implementation of USP <382> on December 1st, 2025. The company’s experts are ready to address pharmaceutical companies’ concerns and support the seamless transition to this new regulatory landscape, so that they can focus on drug development and bring safer therapeutic options to patients around the world.

