To Issue 175
Citation: Nightingale A, “CDMOs as Strategic Enablers for Device Industrialisation”. ONdrugDelivery, Issue 175 (Jul 2025), pp 20–24.
Adam Nightingale explores a multitude of approaches to achieving more efficient and sustainable production of medical devices when partnering with a CDMO, and demonstrates the ways in which these partnerships can offer reduced risk and comply with regulations when streamlining industrialisation.
The medical device industry is larger and more innovative than ever. Clinical research is experiencing a surge of innovation in life sciences, with breakthroughs such as cell and gene therapies, personalised medicines, identification of new biomarkers and point-of-care diagnostics. These technological advances not only create exciting opportunities for innovative product development but also introduce new complexities that demand a sophisticated approach to device manufacturing.
Global challenges such as navigating an increasingly complicated regulatory landscape, an overall global lack of manufacturing capacities and high levels of geopolitical uncertainty only add to the complexity of industrialisation. Successfully getting a medical device to market requires an integrated strategy for design and manufacture, which manages risk at each stage of the development process. In this context, the right CDMO is more than a vendor – it can and should be a strategic enabler.
“THE NEED TO PRODUCE SMALLER BATCHES OF DEVICES FOR CLINICAL TRIALS IS BECOMING INCREASINGLY DIFFICULT, AS THE CAPABILITIES REQUIRED IN TERMS OF AGILITY OFTEN DIFFER FROM THOSE FOR HIGH-VOLUME PRODUCTION.”
GLOBAL CAPACITY AND FLEXIBLE MANUFACTURING
One of the most critical assets in today’s medical device landscape is manufacturing capacity and a flexible global footprint. The surge in demand for glucagon-like peptide 1 (GLP-1) agonists has led to a massive demand for manufacturing capacity from big pharma companies for the devices that deliver them. In turn, this has created a shortage of capacity for other medical device companies. In particular, the need to produce smaller batches of devices for clinical trials is becoming increasingly difficult, as the capabilities required in terms of agility often differ from those for high-volume production.
Uncertainty regarding the geopolitical situation also implies that medical device companies need to be prepared to be globally flexible. Having partners who can offer suitable manufacturing capabilities in different parts of the world offers resilience as well as improved access to markets. With its worldwide geographical footprint, Sanner Group provides these capabilities and can fulfil diverse customer needs – from regional market launches to globally distributed product lines. Additionally, all manufacturing sites implement the same high-quality approaches and meet the latest global standards such as current GMP, ISO 13485 and ISO 9001 (Figure 1).

Figure 1: Sanner has the global manufacturing capacity to fulfil diverse customer needs.

Figure 2: Modular bays allow for quick production ramp-up.
CLINICAL MANUFACTURING – CRUCIAL FOR DEVICE COMMERCIALISATION
Clinical builds are critical to the success of a novel product. Device companies and their partners must be prepared to react quickly to new developments, clinical trial findings or regulatory changes. Also, they must have suitable manufacturing systems in place to avoid long set-up delays, which might lead to commercial opportunities being missed. This is where Sanner’s new headquarters in Bensheim, Germany, comes into play. The state-of-the-art 10,000 m2 production facility is designed to be especially scalable and adaptable (Figure 2). The facility features modular bays to support both injection moulding and automated assembly, while also remaining easily reconfigurable based on project needs. Expansion areas are already in place for cleanroom production, high-precision injection moulding and complex automation lines, enabling a quick ramp-up without disruption.
By integrating clinical insights with technical expertise, Sanner can help transform designs into clinically validated solutions positioned for regulatory success and market acceptance. Dedicated experts can identify and resolve design limitations that might impact clinical outcomes. This includes implementing targeted modifications to enhance device functionality, address performance gaps and ensure compliance with evolving clinical requirements. Whether preparing for pivotal trials or addressing post-market performance expectations, cross-functional teams can bridge the gap between innovative concepts and clinical reality. Through iterative prototyping and manufacturing simulations, Sanner can create designs that balance clinical effectiveness with production efficiency. This dual focus reduces clinical trial risks while establishing robust processes for commercial-scale manufacturing.
MANAGING COMPLEXITY WITH INTEGRATED DESIGN AND MANUFACTURING
Developers of medical devices must consider a wide range of factors, including clinical functionality, usability, manufacturability, cost-effectiveness and regulatory requirements. When these considerations are addressed in silos – by different teams and at different times – it can result in an inefficient process with costly redesigns, project delays and elevated levels of technical risk.
Mapping this entire process out as a single programme with an integrated team can provide the foundation for a successful project. From early feasibility and human-centric design through to high-volume serial production, assembly and packaging, each step of the process needs to feed seamlessly into the next. This is achieved by embedding capabilities such as design for manufacturing and assembly, human factors engineering, and regulatory affairs into a development project right from the beginning. Early collaboration can help to avoid late-stage surprises, which can derail timelines and compromise product viability. Hence, building long-term feasibility and scalability into the design itself, instead of adding them as an afterthought, is paramount.
With Design Centres of Excellence in the high-tech hubs of Cambridge, UK, and North Carolina, US, Sanner has access to the highest level of design and development expertise, along with the unique ability to transfer this into cutting-edge medical device manufacturing completely in-house to the manufacturing facilities on three continents.
ACCELERATING INDUSTRIALISATION THROUGH PROTOTYPING
Industrialisation – the process of turning a design into a production-ready product – is often the phase in which projects stall. As already mentioned, the design and product concept must be suitable for reliable high-volume manufacturing from the beginning of the project. Here, iterative prototyping offers a way to reduce technical risk before incurring the high capital investments required for industrialisation.
Technologies such as 3D printing are well-suited for early prototypes. However, representative prototypes of production devices are likely to require pilot moulding. Sanner uses an innovative proprietary change mould system for rapid turnaround of moulded parts and can even 3D-print ceramic tool inserts for “real” parts in a matter of days.
“AUTOMATION SHOULD BE DESIGNED TO MEET THE REQUIREMENTS OF THE DEVICES AND THE INCREASING COMPLEXITY, THE MORE PARTS THAT MUST BE ASSEMBLED.”
ADVANCED AUTOMATION FOR QUALITY AND EFFICIENCY
Another aspect that is crucial for efficient industrial-scale manufacturing is automation – not just for speed and cost efficiency, but also for ensuring consistent quality. Automation should be designed to meet the requirements of the devices and the increasing complexity, the more parts that must be assembled. Increased repeatability and the reduction of human errors combined with a higher degree of in-process controls, such as load cells or camera checks, make the highest levels of quality assurance possible.

Figure 3: The use of AGVs significantly automates handling and logistics.
Using technologies such as automated guided vehicles (AGVs) to transport containers to and from production areas minimises manual handling can reduce the risk of contamination and ensure a clean, safe environment for medical device manufacturing (Figure 3). Automated warehouse systems can optimise logistics and enable efficient inventory management, which is key to ensuring full traceability for a product. Sanner additionally uses virtual reality simulations to enhance the design for assembly process, generating early insights and supporting proof-of-principle studies. This innovative approach optimises seamless scalability to industrial volumes with shortened timelines.
GLOBAL SUPPLY CHAINS AND REGULATORY COMPLIANCE
Supply chain disruption has become one of the defining challenges of recent years. From raw material shortages to transportation delays, these issues can have huge impacts on launch timelines and production continuity. For any global medical device supplier, it is vital to analyse the resilience of its manufacturing supply chains and put in place suitable mitigations to the biggest risks. As such, Sanner has established a global injection moulding backup system for medical devices. Through a combination of multi-sourcing strategies, local supplier networks, and digital supply chain monitoring, the company offers both agility and security. In addition, Sanner’s global footprint allows it to shift production as needed to respond to changing conditions or customer requirements.
At the same time, regulatory compliance and navigating regulatory approval is a core part of any medical device project. Risk mitigation should be proactive, and regulators will expect to see a clear link between the customer requirements and design inputs from the development process and the controls in place during manufacturing. When targeting multiple global markets, a coherent strategy is vital. Sanner has the expertise across Europe, North America and China to support customers through ISO 13485 audits, prepare documentation for CE marking or ensure FDA 21 CFR Part 820 compliance.
FOCUS ON SUSTAINABLE MATERIALS AND PROCESSES
Customers are placing increasing demands on sustainable manufacturing, with sustainability goals now top-level business objectives for multinational medical device companies. Managing this alongside the requirement for many medical devices to be disposable is a considerable challenge wherein both the design of medical devices and their manufacturing must be considered.
Much of the environmental impact of a device is fixed at the design stage by early decisions taken about the product architecture, materials and number of parts, among other factors. Sanner’s sustainable design experts apply techniques such as lifecycle analysis to reduce the environmental impact of a design whilst still meeting all user needs. This holistic approach to minimising environmental impact brings in resource efficiency, chemical compatibility and possible alternative materials, as well as the environmental and social impact of raw material extraction, refining, transport and end-of-life disposal. Construction and shape are optimised wherever possible to utilise less resources, and Sanner offers a range of bio-based polymers suitable for some applications.
When it comes to manufacturing, new technologies are reducing the environmental impact of factories and production processes. For example, Sanner’s newly expanded Bensheim site features an 18,000 m2 roof entirely covered with photovoltaic panels, supplying renewable energy to operations. Beneath the facility, a green oasis provides biodiversity support and natural cooling, helping to regulate the building’s temperature in an eco-friendly way. Additionally, three wind turbines supplement the energy mix, ensuring greater independence from fossil fuel sources. These initiatives earned Sanner a Bronze rating from EcoVadis in 2024.

Figure 4: The SmartSite™ IV bag is a good example for sustainable development.
PROOF OF CONCEPT: REAL-LIFE CASE STUDIES
The SmartSite™ IV bag and the needle-free SmartSite™ valve connector, developed by Sanner’s US design and development team in close partnership with a key customer, are good examples of sustainable development (Figure 4). The solution addresses the need for empty IV bags, predominantly in in-patient care. As needlestick injuries are a frequent source of injury in hospitals, the bag has a needle-free valve, which eliminates the risk of needle-stick injury during handling. The bags also offer a significant environmental advantage – thanks to their specific design, they can be filled with IV solution, hygienically disinfected and refilled with new medication. This reduces single-use plastic waste considerably. Additionally, they are designed to collapse when emptying to minimise drug waste and save even more resources.
Another example of holistic device industrialisation is the next generation Sentimag system from Endomag, which is used to guide surgeons to the site of breast cancers during surgery. It includes a Sentimag Smartprobe Slim™, which has 50% smaller volume, allowing for easier access and greater visibility, and was developed by the Sanner subsidiary Springboard (Figure 5). This included determining initial feasibility on how to reduce the volume of the probe without compromising the magnetic sensing performance. Following this, different probe concepts were explored around the overall probe size and shape. Springboard produced prototypes of the chosen concept for evaluation by Endomag and then moved to design for manufacture. Production-equivalent probes served for verification testing. The system was approved by the US FDA in January 2023. This case study shows how Sanner can support clients from development to design transfer and beyond. In the future, the company can also produce these devices in its new state-of-the-art manufacturing sites in Europe and the US.
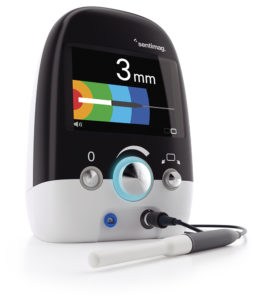
Figure 5: Springboard developed the Sentimag Smartprobe Slim™ for Endomag’s next generation Sentimag system.
CDMOs AS STRATEGIC ENABLERS FOR DEVICE INDUSTRIALISATION
The pressure on the medical device industry is intensifying – from manufacturing shortages, global geopolitical uncertainty and supply chain risks to an increasingly complex regulatory landscape. By combining world-class design expertise, global manufacturing capacity, regulatory insight and operational excellence, Sanner helps its partners not only to navigate these challenges but to thrive within them.
Whether launching a next-generation combination product with a new drug delivery device, an innovative diagnostic or a connected health device, Sanner provides everything pharmaceutical companies need from a single source. As healthcare continues to evolve, so too must the partnerships that drive it. With a foundation of quality and shared purpose, Sanner is ready to meet the future of medical device development together with its customers and partners.

