To Issue 176
Citation: Ciccarelli N, King A, Herrmann M, Roschke T, “Accelerating Development of Electromechanical Delivery Systems via Modular Integration”. ONdrugDelivery, Issue 176 (Sep 2025), pp 58–64.
Nicholas Ciccarelli, Andrew King, Dr Marcus Herrmann and Dr Thomas Roschke, discuss the insights gained in the product development process from the recent collaboration between the two companies, with Kymanox managing the overall system integration and Johnson MedTech tackling the specific design of the drive system within the designated product specification.
Reusable, electromechanical drug delivery systems are becoming increasingly sought after for their superiority over their disposable counterparts, specifically for their increased sustainability and precision dosing. At the heart of many advanced drug delivery systems lies a precision motion subsystem – a custom motor and gearbox designed to deliver exacting performance in a compact, long-lasting package.
This article explores the system integration of highly customised subsystems through a collaborative partnership between Kymanox, an expert in drug delivery device design and development, and Johnson MedTech (JMT), a specialist in miniaturised dosing and needle insertion systems. Both companies embraced a collaborative development approach, wherein Kymanox defined clear interfaces and performance criteria and JMT was free to engineer the internal workings. With that, both companies were able to accelerate development and deliver innovative solutions.
The clarity of the proper technical interfaces was the result of this joint co-operation, leading to a mutual understanding of the drug delivery and motion system needs. One of the most impactful outcomes of this approach was the ability to test and verify the subsystem independently from the final product, significantly reducing risk and increasing efficiency.
INTERFACE DEFINITION: FOUNDATION FOR MODULAR DEVELOPMENT
The cornerstone of this collaboration was the thoughtful definition of the interface between the drug delivery device (Kymanox module) and the motor-gearbox subsystem (JMT module). Kymanox’s goal was to precisely define what the subsystem needed to achieve – without dictating how it was achieved. This interface definition typically includes:
- Mechanical Parameters: Mounting geometry, shaft alignment, space envelope and tolerance allowances.
- Electrical Requirements: Supply voltage range, current limits and connector standards.
- Performance Expectations: Speed and torque profiles, positional accuracy, response times and lifecycles, as well as environmental conditions.
This modular approach enabled JMT to treat the subsystem as an independent, clearly bounded unit. JMT had full control over the internal design, while ensuring compatibility with the broader device. Simultaneously, JMT provided feedback to Kymanox on how those expectations could be achieved in the most efficient way. Within a few paper-based iterations, the proper system boundaries were able to be clarified and refined. To get the most out of both engineering teams, those boundaries were elaborated on during each system iteration, based on the specific application conditions and requirements.
“RATHER THAN OVER-PRESCRIBING SUBSYSTEM REQUIREMENTS TO AN INDUSTRY EXPERT, KYMANOX FOCUSED ON DESIGNING THE OVERARCHING SYSTEM ARCHITECTURE, ORCHESTRATING MULTIPLE SPECIALISED PARTNERS, BRINGING IN USER AND PATIENT EXPERIENCE AND INTEGRATING ALL COMPONENTS AND SUB-SYSTEMS.”
KYMANOX’S ROLE: SYSTEM INTEGRATION EXPERTISE IN ACTION
Kymanox brought deep experience in system integration to the table – a strength that proved essential in defining subsystem boundaries and ensuring seamless incorporation into the complete device. Rather than over-prescribing subsystem requirements to an industry expert, Kymanox focused on designing the overarching system architecture, orchestrating multiple specialised partners, bringing in user and patient experience and integrating all components and sub-systems. This effort included:
- Identifying Critical Parameters: Knowing which aspects of the motion system truly matter for overall device performance and reliability.
- Clarifying Functional Interfaces: Defining the mechanical and electrical connections in ways that are robust yet flexible.
- Orchestrating Parallel Development: Co-ordinating efforts across vendors, ensuring that the timeline moves forward without bottlenecks.
By clearly articulating requirements and constraints while trusting experts such as JMT to solve within them (Figure 1), Kymanox ensured that the system-level goals were met without micromanaging the implementation.
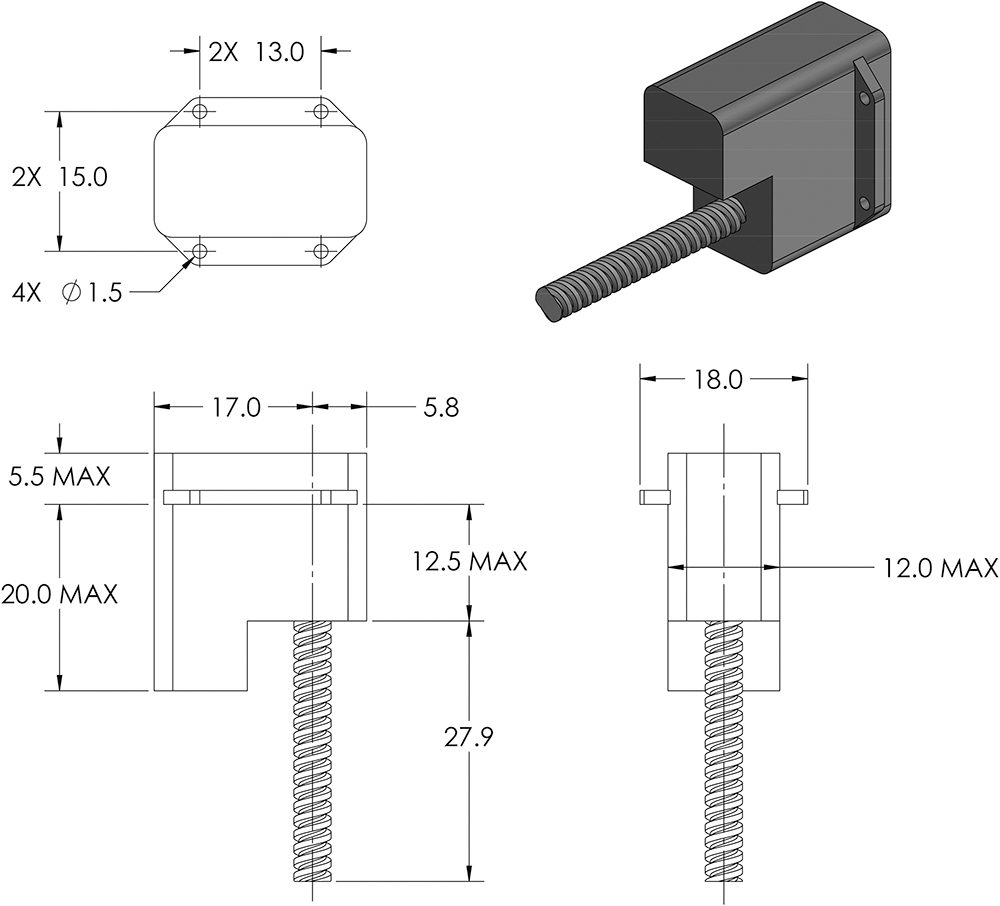
Figure 1: Example of a design envelope from Kymanox for an actuator module showing dimensional maxima, mounting locations and interface points.
JMT’S ROLE: ENGINEER THE “HOW”
Within the agreed boundaries, JMT had full freedom to develop the optimal motor-gearbox-sensor solution. Rather than being constrained by prescribed components or legacy configurations, JMT was able to explore and simulate various design pathways to meet the performance requirements.
Simulation-Driven Design Process
JMT used a suite of advanced tools to optimise the subsystem virtually before committing to physical prototypes:
- Mathematical Modelling: Simulating how the motor would perform under load and adjusting parameters such as winding configuration and magnetic characteristics.
- System Simulation: Evaluating the overall efficiency, response time and duty cycle compliance to ensure good battery life and dosing precision during the different delivery profiles.
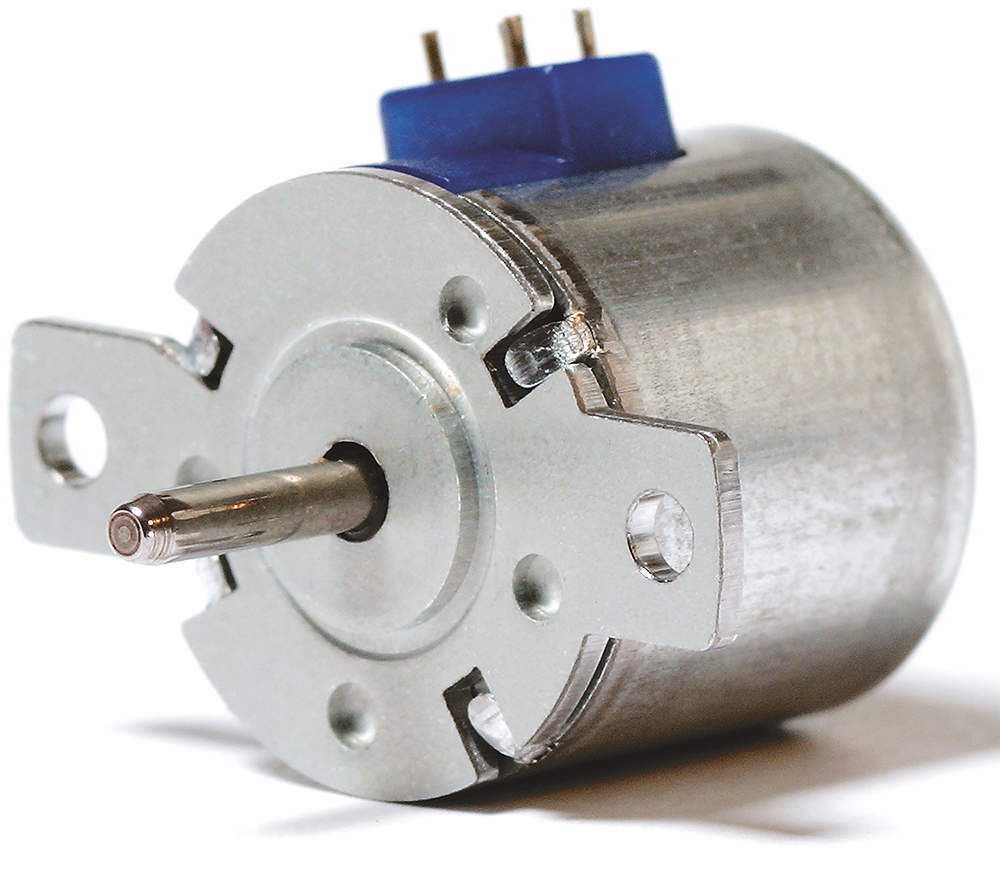
- Selection of the Best Fitting Motor Principle: Determining if brushed motors, stepper motors or electronically commuted motors are the best fit for the required drive train (Table 1).
- Finite Element Analysis (FEA): Studying gear stress, deformation and long-term durability under cyclic loading.
To identify the optimal solution within the given design envelope, JMT investigated a variety of alternative motor technologies and geartrain options. The selected motor principle is one of the base decisions in drive-train design and must be based on the requirements of the application. Common motor technologies are shown in Table 1, together with their key parameters.
| Characteristic | Brushed PMDC Motor | Stepper Motor | Electronically Commutated Motor (BLDC / EC Motor) |
Piezo Motor |
| Reference View | 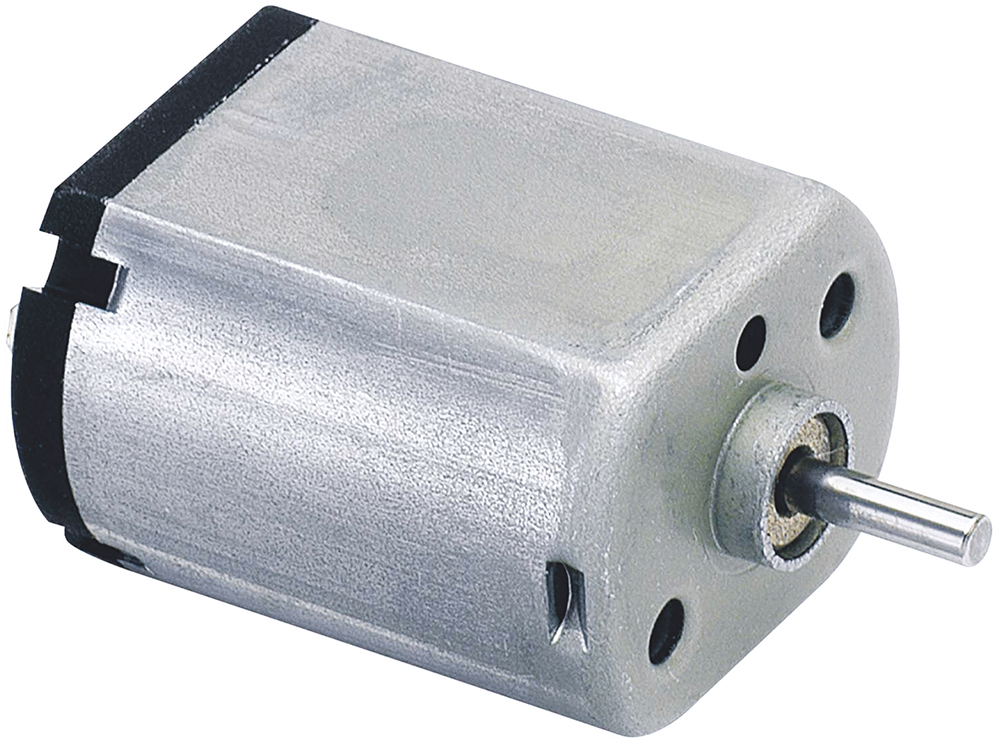 |
 |
 |
 |
| Size | Ø 4–80 mm | Ø 6–42 mm | Ø 4–60 mm | Ø 8–15 mm |
| DC Supply | 1–240 V | 3–48 V | 3–240 V | 5–120 V |
| Performance Characteristics (example) | 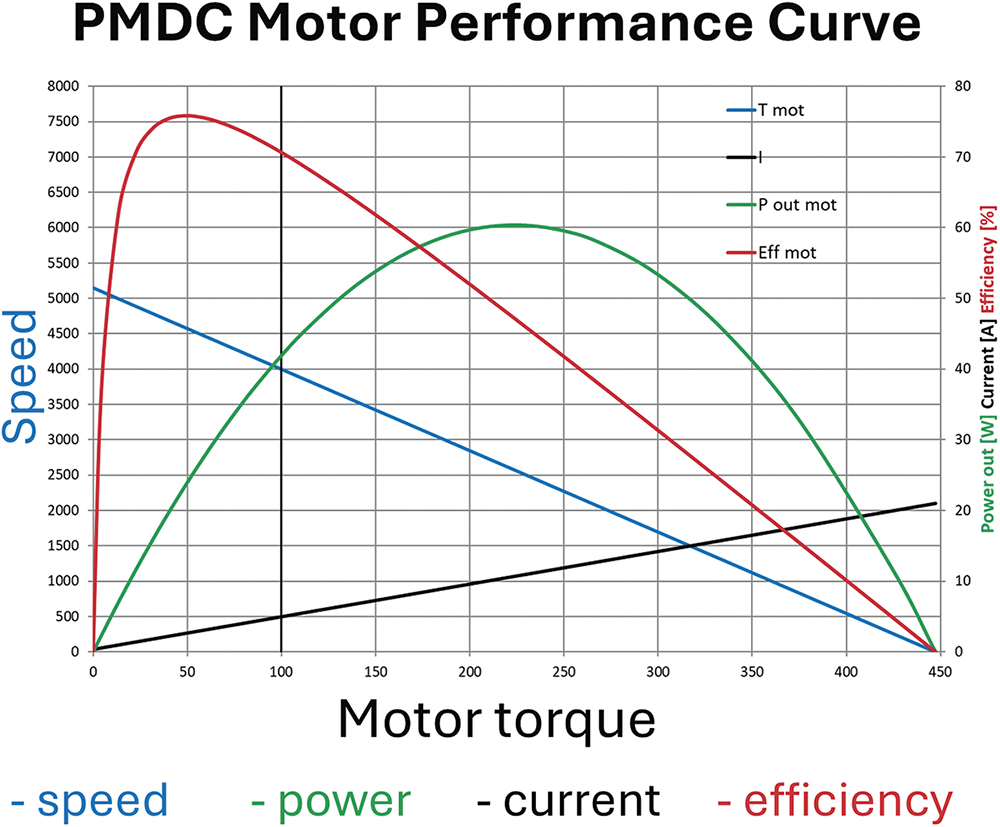 |
 |
 |
 |
| Operational Life | 300–8,000 hrs | 10,000–25,000 hrs | 10,000–40,000 hrs | 20,000–50,000 hrs |
| Resolution | Depending on sensor and control | Tin can 7.5°–18° Hybrid 0.36°–3.6° |
Depending on sensor and control | Depending on sensor and control (e.g. 4096 CPR) |
| Reliability | Mainly limited by electrical commutator | Mainly limited by electronic control circuit and bearing |
Mainly limited by electronic control circuit and bearing |
Mainly limited by tip contact and electronic control |
| Sensors | Can be added | Can be added | Built-in hall sensors or similar | Built-in hall sensors or similar |
| Cost | Lowest | Low to medium | Medium | High |
| Operation Temperature | -20°C – +60°C | -40°C – +80°C | -40°C – +80°C | -40°C – +70°C |
| Notes | Rotary and linear versions available | Rotary and linear versions available |
Table 1: Comparison of the common features of miniature brushed PMDC-motors, steppers, electronically commutated motors and piezo motors.
As an example, for low speed, high torque applications, a stepper motor is often preferable, featuring open loop control possibilities and precise dosing accuracy. With that selection, the need for additional sensors might be avoided, reducing cost and complexity. On the other hand, it may be preferable to use a direct current (DC) motor for continuous dosing, having the benefit of low power consumption and high efficiency. Both motor principles require a custom gear train design according to their characteristics. Reliability, operational life or related costs are also often driving factors in the selection of the actuation solution.
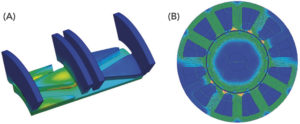
Figure 2: (A) Magnetostatic field analysis of a small stepper motor (ANSYS); (B) Magnetostatic field analysis of a high pole BLDC motor (MotorCAD).
To clearly determine the best technology choice and get Kymanox’s buy-in on the proposed solution, JMT used mathematical simulation tools to predetermine performance and life, thereby demonstrating feasibility. FEA and simulations were used for motor performance (Figure 2), as well as dynamic system simulations using either Matlab, Simulink, SimScape or SimulationX (Figure 3). For example, simulated data of motor currents (Figure 3, Right) was typically shared with Kymanox to support their early controller design for drug delivery devices.
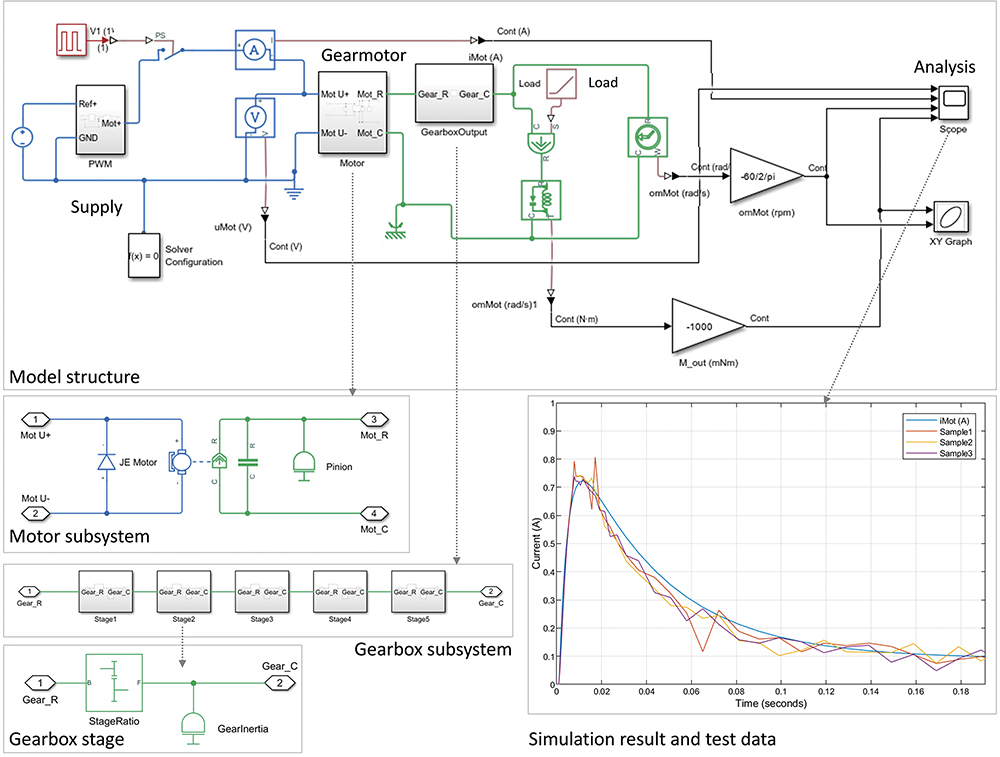
Figure 3: Dynamic simulation model for a PMDC Motor with a multi-stage gearbox (Matlab/Simulink/SimScape) and its simulation result for the motor current during start of operation in comparison to prototype test data.
“THE INHERENT DESIGN TRADE-OFF IN DOSING SYSTEMS IS BETWEEN SIZE AND PERFORMANCE – A SMALLER SIZE IS A KEY CONTRIBUTOR TO PATIENT CONVENIENCE, YET SMALLER COMPONENTS ARE NATURALLY MORE FRAGILE.”
The inherent design trade-off in dosing systems is between size and performance – a smaller size is a key contributor to patient convenience, yet smaller components are naturally more fragile. Furthermore, an increasing number of pharmaceutical companies are commercialising higher viscosity drugs that, when coupled with fast injection times, create higher forces inside the geartrain. JMT used advanced analysis software, such as KissSoft and ANSYS, to predict the robustness and endurance of geartrains with respect to the expected device lifespan (Figure 4).
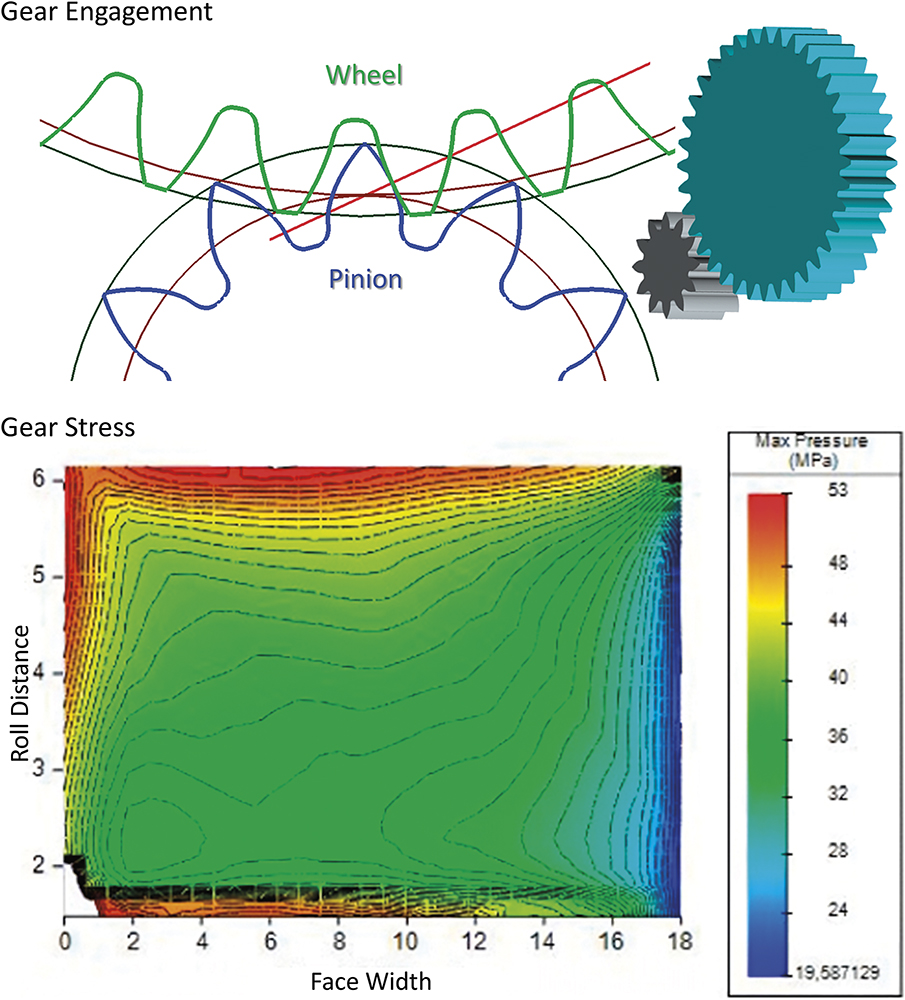
Figure 4: Gear profile optimisation and gear stress simulation (KissSoft).
The robustness of the system is determined not only by mechanical loads due to external forces but also by the influence of tolerances – manufacturing tolerances play a major role in the final product performance, especially for small components. To evaluate the assembly interface variations, JMT performed statistical tolerance analyses, also by means of computer-aided engineering software. Eventually, this early-stage analysis on the boundary features gave Kymanox important input for the overall system design.
In summary, these tools allowed JMT to rapidly iterate and test ideas in order to hone the design before any parts were manufactured. The benchmark for all these activities was the actuator module boundary requirements, which were incorporated into a comprehensive verification plan, the results of which were exchanged with Kymanox.
Independent Subsystem Testing: A Major Advantage
One of the most significant benefits of this modular approach is the ability to test and verify the motor-gearbox subassembly completely independently from the final device.
- Early Verification: JMT built test rigs tailored specifically to the subsystem, allowing them to assess critical performance characteristics – such as torque, speed performance and thermal behaviour – well before device-level integration.
- Parallel Development: While JMT refined the motion subsystem, Kymanox was able to continue development on other parts of the device. This independent yet collaborative process significantly shortened the development timeline.
- Reduced Risk: By confirming standalone subsystem performance upfront, integration challenges were minimised and system-level testing became more of a confirmation step than a debugging phase.
This subsystem testing approach not only improves development speed but also contributes to increased reliability and confidence in the final product. A major benefit of the independent testing scheme is the possibility to test for failure by exaggerating load profiles or cycles. Additionally, various application conditions can be recreated much faster and more easily, gleaning deeper insights than would be achieved with complete device tests. Figure 5 shows a representative motor-gearbox actuator test bench that enables a variety of tests while collecting precise data. The testing is not done in isolation, but results are compared with the upfront simulations to improve the models and gain more knowledge (Figure 3). By sharing the subsystem results early on, JMT provided reliable data to Kymanox that helped its engineers to refine the delivery device design and to advance system development.
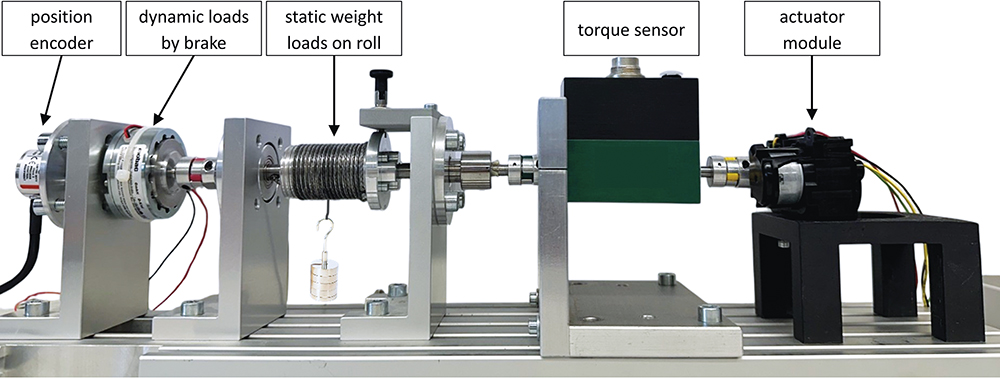
Figure 5: Test bench for a motor-gearbox system used in the modular testing scheme (controller part not shown).
RESULTS: HIGH PERFORMANCE WITHIN A CLEAR BOUNDARY
With a collaborative approach, the resulting motor-gearbox-sensor subsystem delivered on all fronts:
- Required specifications were met, with speed and torque profiles optimised to the use case
- The components fit seamlessly within the device envelope without compromising other design goals
- The drive system was verified as a standalone unit, ensuring readiness for integration with minimal reworks.
The disciplined separation of responsibilities and the precise definition of interfaces made this possible, allowing each party to contribute with its core strengths and expertise. In the long term, this definition also allowed for a clear monitoring and revalidation of the motion subsystem’s performance during the device’s production life.
CONCLUSION: CLEAR BOUNDARIES, BETTER OUTCOMES
The collaboration between Kymanox and JMT highlights a powerful model for medical device development using integrated drive systems – define what is needed, then let subsystem domain experts decide how to deliver it. By focusing on the interface and giving the subsystem supplier design freedom, the design teams were able to unlock higher-quality solutions, faster development and lower integration risk.
Above all, however, a shared technical understanding of the dosing systems’ function remains essential. The complexity of wearable drug delivery devices requires clear technical specification boundaries and frequent communication. By doing so, independent subsystem testing becomes feasible and effective, enabling early issue detection and smoother final assembly.
As drug delivery devices and medical devices continue to increase in complexity and sophistication, such modular, interface-driven collaboration models are not just beneficial – they are essential. With Kymanox’s strength in system integration and JMT’s technical depth in electromechanical subsystems, this partnership shows how modern engineering is as much about collaboration as it is about innovation.

