To Issue 180
Citation: Rogueda P, “Inhaled Oligonucleotides – the Future for Respiratory Diseases”. ONdrugDelivery, Issue 180 (Nov 2025), pp 68–72.
Dr Philippe Rogueda discusses the therapeutic potential promised by oligonucleotides and presents the case for prioritising the inhalation route as the ideal delivery mechanism for these drugs, highlighting how direct delivery to the lungs via devices such as dry power inhalers or soft mist inhalers can maximise the therapeutic efficacy and minimise the side effects of these exciting drugs.
In the vast universe of the human genome, a small fraction – only about 1.5% – is dedicated to coding for proteins, the building blocks and machinery of our cells. Of these proteins, only a minority have active sites that can be targeted by traditional medications, leaving a vast number of diseases beyond the reach of conventional therapies.1 This limitation has ushered in the era of oligonucleotides, a revolutionary class of therapeutics that promises to unlock new treatments by targeting previously inaccessible proteins within our genetic material.
Oligonucleotides, short sequences of synthetic DNA or RNA, are designed to interact with our genetic machinery, allowing for the precise modulation of gene expression. By either blocking harmful proteins from being made or correcting genetic messages, these molecules offer a new pathway to treat a myriad of conditions. With the potential to target over 10,000 proteins, oligonucleotides represent a leap forward in medicine, providing hope for diseases once deemed untreatable.
The journey of oligonucleotides from concept to therapeutic reality has not been without challenges, especially in delivering these fragile molecules to the right part of the human body. This article focuses on one of the most promising avenues for oligonucleotide delivery – inhalation into the lungs. Direct lung delivery offers a non-invasive route to treat respiratory conditions, maximising therapeutic effects while minimising systemic side effects.
OLIGONUCLEOTIDES FOR RESPIRATORY CONDITIONS
Oligonucleotide therapies have shown remarkable efficacy against respiratory conditions through their mechanism of modulating intracellular gene expression. There are currently 71 oligonucleotide molecules in the development pipeline for respiratory conditions, and the diseases they target can be grouped into three distinct categories:
- Genetic disorders, such as cystic fibrosis (CF)
- Inflammatory diseases, such as asthma and COPD
- Lung infections, such as coronavirus infections.
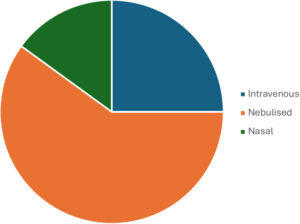
Figure 1: Routes of administration for oligonucleotides intended for respiratory conditions.
To date, the majority of oligonucleotides for respiratory conditions in development are delivered parenterally or through inhalation (Figure 1).2 An important aspect of maximising the therapeutic effect of the oligonucleotide – with or without the appropriate delivery cargo – is the identification of an efficient route of administration. Parenteral administration presents numerous challenges to naked oligonucleotide delivery,3 including:
- Short half-life due to rapid nuclease degradation4
- Rapid clearance from the kidney due to small size4
- The need to cross cellular barriers
- Unwanted side effects
- Risk of needlestick injuries and blood-borne diseases
- The requirement of trained personnel for administration
- Sterile formulations.
Another interesting challenge is that oligonucleotides can accumulate in off-target tissues following parenteral administration, but local delivery mitigates this problem. Many side effects can be minimised with a lower dose of oligonucleotides, and local delivery allows for lower doses. In the case of respiratory infections and lung diseases, pulmonary delivery would allow the optimal dose to be delivered directly to the target tissue, minimising side effects and accumulation in off-target tissues.
“WHILE IT HAS CLEAR AND POWERFUL BENEFITS, DIRECT DELIVERY OF OLIGONUCLEOTIDES TO THE LUNGS IS NOT WITHOUT ITS CHALLENGES.”
DELIVERING OLIGONUCLEOTIDES DIRECTLY TO THE LUNGS
While it has clear and powerful benefits, direct delivery of oligonucleotides to the lungs is not without its challenges. Mucociliary clearance, for example, is a protective mechanism by which inhaled particles are removed from the lungs. This is a rapid process, meaning that the overall time available to inhaled RNA for absorption is restricted, and has been shown to trap and promote the removal of RNA delivery vectors to great effect, therefore presenting a formidable barrier to inhaled oligonucleotide delivery.5
Moreover, when dealing with lung diseases, the mucosal barrier is often more difficult to bypass when compared with a healthy state. For example, in CF and COPD, the viscosity and elasticity of the mucus is greater, making RNA penetration harder.6 Furthermore, inflammation of the airway can cause mucin hypersecretion, leading to a greater number of mucins available to potentially bind RNA delivery vectors and impede their delivery.7
Delivery vectors can be used to traverse the lung mucus barrier, such as placing the RNA in chitosan nanoparticles, which have mucoadhesive properties – prolonging the contact time between the nanoparticle and mucosal surface, thereby enhancing the absorption of its drug cargo.8 Another delivery strategy is to densely graft polyethylene glycol (PEG) onto the surface of the nanoparticles – a process termed PEGylation. This strategy has been widely investigated to overcome the mucus barrier for several routes of delivery including vaginal, oral, ocular and nasal. Via PEGylation, a hydrophilic and neutrally charged polymer has been shown to effectively enable nanoparticles that would have otherwise been immobilised to diffuse rapidly through lung mucus,9 and its success is well-documented in the literature over the past decade or so.3,6
Another barrier that must be overcome for effective pulmonary delivery of RNA is the presence of alveolar macrophages. These cells operate as part of the immune system to protect the body by engulfing and degrading inhaled foreign macromolecules through phagocytosis.3 The primary method employed for macrophage evasion so far has been particle engineering. The aim has been to produce large, porous particles with a physical diameter of less than 10 µm, making them too big for phagocytosis but leaving them with a small aerodynamic diameter due to their lower density. Hitting this sweet spot represents one option to evade macrophages in the deep alveolar regions.
It is uncommon to deliver “naked” oligonucleotide molecules, as they are unlikely to overcome the challenges associated with each route of administration. Therefore, another consideration is ensuring that the inhalation delivery method can properly aerosolise the carrier vector as well. Potential carrier vectors include:
-
Bioconjugation – covalent binding of oligonucleotide to:
– Lipids (e.g. cholesterol)
– Peptides
– Aptamers
– Antibodies
– Sugars (e.g. N-acetyl galactosamine) - Nanocarriers:
– Liposomes
– Exosomes
– Spherical nucleic acids
– DNA nanostructures.
SELECTING THE RIGHT INHALATION DELIVERY DEVICE
Beyond engineering the oligonucleotide structure and delivery vector, an important consideration is selecting the right delivery device for inhaled oligonucleotide therapies. To date, there are several platforms for effectively delivering oligonucleotide therapies to the lungs, and each platform has its own advantages and disadvantages.
Dry Powder Inhalers
Dry power inhalers (DPIs) are propellant-free, portable and easy-to-use inhalation devices. They store drug formulation as a dry powder, typically in capsules, blisters or a reservoir, and rely on the patient’s inspiratory effort to aerosolise the powder and deliver it into the upper respiratory tract. However, one of the concerns in the powder formation of nucleic acids is their stability – individual powder-forming techniques involve several physical treatments, such as heating, agitation, atomisation, freezing and drying, some or all of which may reduce the structural and functional integrity of nucleic acids under physical stresses, such as shear force and extreme temperatures.
To mitigate the destabilisation of nucleic acids during powder formation, non-viral vectors, such as cationic lipids and polycations, have commonly been included as powder components for their stabilising effects, as well as their high transfection efficiency through electrostatic complexing.10 However, from the viewpoint of safety, it is also desirable to produce the powders of naked nucleic acids without using vectors.10–12 Merxin Ltd’s capsule-based DPI, MRX003, and multidose DPI, MRX006, are ideally suited for the delivery of oligonucleotide formulations. MRX003 is already available on the European market, trusted by prescribers and patients alike for its reliability and ease of use. MRX006, designed for advanced therapeutic applications, supports triple combination therapies and enables the separation of two or more incompatible formulations through its dual blister strip mechanism.
“SMIs ALLOW FOR A SLOWER RELEASE OF AEROSOL COMPARED WITH TRADITIONAL INHALERS, RESULTING IN A WEAKER DESTRUCTIVE EFFECT ON DRUGS. THIS GENTLER APPROACH IS IDEAL FOR DELIVERING OLIGONUCLEOTIDES.”
Soft Mist Inhalers
A soft mist inhaler (SMI) is a convenient, portable inhalation device that delivers the drug as a fine, slow-moving mist. To achieve this, a spring provides mechanical energy that forces a solution containing the API through a fine nozzle, producing a particle cloud or soft mist, which allows relatively easy inhalation deep into the lung. SMIs allow for a slower release of aerosol compared with traditional inhalers, resulting in a weaker destructive effect on drugs.13 This gentler approach is ideal for delivering oligonucleotides.
For example, in one study by Wang et al, a small interfering RNA (siRNA) loaded in a polymer lipid nanoparticle intended for lung cancer treatment was aerosolised using a commercially available SMI. The authors measured changes in particle size and polydispersity index (PDI) to determine the physical stability of the siRNA molecules after aerosolisation via the SMI. The particle size had a slight increase (approximately 10 nm) and the PDI also increased – although it remained under 0.26, indicating that the particle sizes were still uniform. Importantly, the nanoparticles loaded with siRNA showed good physical stability when aerosolised via the SMI.14
In a direct comparison of SMIs with nebulisers, Miao et al compared the delivery of lipid nanoparticles (LNPs) containing mRNA using different atomisation methods:
- Two fluid nozzle
- Jet nebuliser
- Ultrasonic nozzle
- SMI
- Vibrating mesh nebuliser.
The authors found that the SMI was a softer atomisation method than the vibrating mesh nebuliser. According to transmission electron microscopy, the morphologies of the LNPs were maintained after the SMI aerosolisation; however, the LNPs tended to be destroyed and reassembled to form the LNPs with larger size distribution after vibrating mesh nebulisation. Additionally, the entrapment and transfection efficiencies of the LNPs were superior after the SMI atomisation compared with after using vibrating mesh nebulisers, as demonstrated by in vivo and ex vivo fluorescence imaging of the RNA after administration of doses. There was approximately a four-fold increase in concentration following SMI administration, compared with the vibrating mesh nebulisers.15 MRX004, Merxin Ltd’s SMI illustrates this perfectly. While delivering three to five times the dose of a traditional nebuliser,its mild aerosolisation process simultaneously protects delicate molecules, such as oligonucleotides, preserving their integrity and optimising therapeutic outcomes.
“FROM OVERCOMING BIOLOGICAL BARRIERS TO SELECTING THE RIGHT INHALATION DEVICE, EVERY STEP IN THE DEVELOPMENT PROCESS PLAYS A CRITICAL ROLE IN ENSURING THERAPEUTIC SUCCESS.”
CONCLUSION
As oligonucleotide therapies continue to redefine the boundaries of respiratory medicine, the importance of effective pulmonary delivery cannot be overstated. From overcoming biological barriers to selecting the right inhalation device, every step in the development process plays a critical role in ensuring therapeutic success. Merxin Ltd’s inhaler devices, MRX003, MRX004 and MRX006 are purpose-built to meet the unique demands of oligonucleotide delivery, offering both protection and precision in administration. For companies developing oligonucleotide molecules, it is critical to consider their choice of delivery route and how delivery of their drug candidates could be enhanced by tailored device technology, such as those offered by Merxin Ltd.
Merxin Ltd Internal Reference: MM-2452-V01
REFERENCES
- Damase TR et al, “The Limitless Future of RNA Therapeutics”. Front Bioeng Biotechnol, 2021, Vol (9), art 628137.
- Gupta A et al, “Nucleic Acid Delivery for Therapeutic Applications”. Adv Drug Deliv Rev, 2021, Vol 178, art 113834.
- Neary MT, “Nebulised Delivery of RNA Formulations to the Lungs: From Aerosol to Cytosol”. J Control Release, 2024, Vol 366, pp 812–833.
- Gao S et al, “The Effect of Chemical Modification and Nanoparticle Formulation on Stability and Biodistribution of siRNA in Mice”. Mol Ther, 2009, Vol 17(7), pp 1225–1233.
- Gizurarson S. The Effect of Cilia and the Mucociliary Clearance on Successful Drug Delivery”. Biol Pharm Bull, 2015, Vol 38(4), pp 497–506.
- Duncan GA et al, “The Mucus Barrier to Inhaled Gene Therapy”. Mol Ther, 2016, Vol 24(12), pp 2043–2053.
- Evans CM, “Mucus Hypersecretion in Asthma: Causes and Effects”. Curr Opin Pulm Med, 2009, Vol 15(1), pp 4–11.
- Yee Kuen C, Masarudin MJ, “Chitosan Nanoparticle-Based System: A New Insight into the Promising Controlled Release System for Lung Cancer Treatment”. Molecules, 2022, Vol 27(2), p 473.
- Huckaby JT, Lai SK, “PEGylation for Enhancing Nanoparticle Diffusion in Mucus”. Adv Drug Del Rev, 2018, Vol 124, pp125–139.
- Wanning S, Süverkrüp R, Lamprecht, A, “Pharmaceutical Spray Freeze Drying”. Int J Pharm, 2015, Vol 488(1), pp 136–153.
- Malamatari M et al, “Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation”. Processes, 2020, Vol 8(7), p 788.
- Arpagaus C et al, “Nano Spray Drying for Encapsulation of Pharmaceuticals”. Int J Pharm, 2018, Vol 546(1), pp 194–214.
- Komalla V et al, “Advances in Soft Mist Inhalers”. Expert Opin Drug Deliv, 2023, Vol 20(8), pp 1055–1070.
- Wang H et al, “Tunable Rigidity of PLGA Shell-Lipid Core Nanoparticles for Enhanced Pulmonary siRNA Delivery in 2D and 3D Lung Cancer Cell Models”. J Control Release, 2024, Vol 366, pp 746–760.
- Miao H et a’, “Optimization of Formulation and Atomization of Lipid Nanoparticles for the Inhalation of mRNA”. Int J Pharm, 2023, Vol 640, art 123050.

