To Issue 182
Citation: “Interview with Dr Carolina Canapè and Roger Lüscher: Enhancing Clinical Trials with CliniPilot® and Clear to Clinic™ Programme”, ONdrugDelivery, Issue 182 (Jan 2026), pp 16–15.
Visit Ypsomed at Pharmapack Paris! – Stand 4D26
Figure 1: CliniPilot smart add-on device for Ypsomate autoinjectors.
Q Today, pharma companies are aiming to accelerate clinical development for faster time to market without compromising on data quality or compliance. With that in mind, how is Ypsomed helping its partners achieve speed and reliability in their injection device programmes?
CC A key way that we’re helping our partners achieve speed and quality is with CliniPilot (Figure 1), which is an add-on device that transforms YpsoMate autoinjectors into smart, connected devices that automatically capture injection data such as time, date and injection outcomes, then sync those data into electronic data capture systems via a companion app that can also be integrated into an ePRO app (Figure 2). Historically, it’s been difficult for clinical trial sponsors to collect reliable objective data on injection, so with CliniPilot, we can massively reduce the need for manual data entry, reduce errors and enable real-time injection data capture.
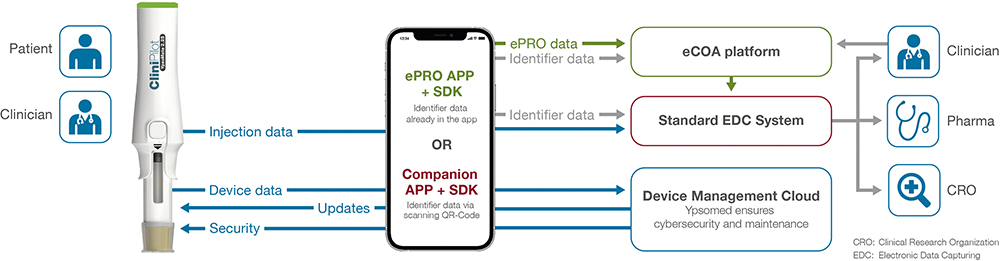
Figure 2: CliniPilot data collection and transfer.
This has many advantages, such as enabling early interventions if, for example, a patient takes their injection at the wrong time. CliniPilot not only improves data quality and trial outcomes but also provides valuable insights about patient behaviour and, critically, this is all done without adding burden to the patients or to the trial site. CliniPilot technology is based on Ypsomed’s SmartPilot, a US FDA-cleared device, and it is suitable for traditional hybrid and decentralised clinical trials models, enabling patients to perform injections at home, minimising the number of necessary visits to the trial site.
“WITH CLEAR TO CLINIC, WE HAVE CLINIC- READY DEVICES THAT ARE ALREADY QUALIFIED AND VERIFIED, MAKING THEM READY FOR USE BY PHARMA PARTNERS.”
Q Next, could you provide an overview of Ypsomed’s Clear to Clinic programme and describe what advantages it offers to pharma companies?
RL So far, Ypsomed has provided customised autoinjector devices for each of our pharma partners, each with its own individual development process – every autoinjector was developed from scratch, which used to take around 12 months. Now, with Clear to Clinic, we have clinic-ready devices that are already qualified and verified, making them ready for use by pharma partners. This approach has reduced project durations to around six months, meaning that autoinjector development is no longer on the critical path in clinical development (Figure 3).
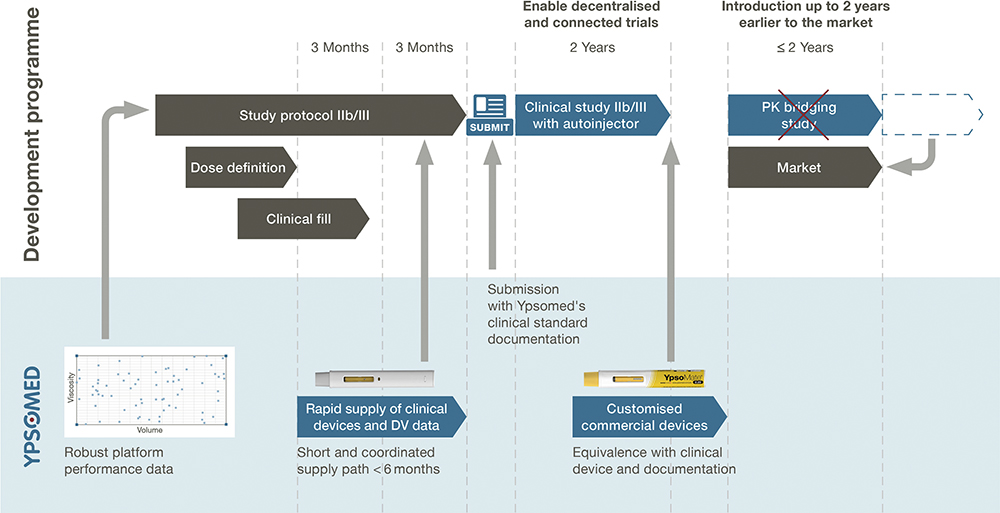
Figure 3: Ypsomed’s Clear to Clinic programme.
Under the traditional model, when autoinjectors are introduced to a clinical study during its later stages, pharma companies would need to conduct a pharmacokinetic (PK) bridging study to demonstrate equivalence between the autoinjector and the vial or safety syringe used in the early trial stages. By taking the pre-developed approach of Clear to Clinic, pharma partners can use autoinjectors in the early trial stages, enabling them to eliminate the PK bridging study and go to market sooner.
With our Clear to Clinic programme, both the autoinjector and the design verification are ready within three months of pharma preparing the clinical fill, which means that they no longer delay the clinical submission. As you know, Phase IIb and III studies are very expensive, so pharma partners want to avoid delays at all costs.
Importantly, our clinic-ready devices cover the full range of fill volumes and viscosities efficiently. This means that a pharma partner can enter a Phase IIb clinical study with a range of fill volumes and pick a device, or even several, to suit their needs. Also, if the fill volume changes from one study to the next, the impact on the device side is relatively minor compared to what it would be with custom device development – they can simply switch to another clinic-ready device.
The last point to make is that the Clear to Clinic programme has a prepared submission dossier that covers the regulatory requirements for early clinical studies. This dossier was put together using our extensive regulatory experience – Ypsomed has contributed to numerous successful combination product submissions over the years. Our regulatory and medical experts are there to provide device-related expertise to our pharma partners from the start of the study protocol through to submission.
Q In your experience, how is connectivity transforming the way clinical trials are designed, conducted and monitored?
CC The adoption of decentralised trial methodologies – combining technologies with in-person site visits – is accelerating across all types of clinical studies. Connected solutions like CliniPilot can be particularly valuable in scenarios where patients face challenges travelling to trial sites. They also play a critical role in long-duration studies, such as those for chronic conditions, which often span multiple years. By enabling seamless connectivity and remote engagement, solutions like CliniPilot help improve patient access, retention and overall trial efficiency.
In summary, CliniPilot connected devices benefit all the stakeholders involved in clinical trials. The shift it enables towards at-home administration benefits patients especially, minimising site visits, eliminating the burden of data collection and improving injection confidence with step-by-step guidance, which has the added benefit of reducing the burden on the medical staff and helping to achieve better patient retention and recruitment. Last, but not least, for pharma, connectivity leads to better, higher quality data that can support faster and better regulatory submissions.
RL What comes to mind for me is an example of a big pharma company that was able to conduct a worldwide study across many patient populations. It would have been a logistical nightmare to perform all the injections within the clinic, so the patients being able to use autoinjectors at home was a major advantage.
Another interesting example to note is rare and orphan diseases. When there are very few patients for a disease, it can be very difficult to find participants for a clinical trial. But, if patients don’t need to go to clinics to participate, it makes recruitment much easier – with at-home administration, the burden on them is much lower, allowing them to fit using the autoinjector in and around their regular lives.
Q How do CliniPilot and Clear to Clinic combine to make clinical trial execution faster and smarter?
CC Put simply, the combination reduces the overall time required for clinical trials. Clear to Clinic gets the autoinjector into clinics sooner and CliniPilot enables trial sponsors to collect objective injection data. On top of that, the data can support the management of the trial itself. For example, if patients are behaving in an unexpected way with self-administration of the drug, the real-time data enabled by connectivity allows clinical teams to intervene quickly and provide adequate support to patients.
Additionally, the data collected from CliniPilot can be linked with other data, such as patient-reported outcomes (ePRO) and clinician-reported outcomes (eCOA). This makes it easier to collect all the patient’s data into a single place for the pharma sponsor, which enable a better, more holistic understanding of the trial data to gain better insights. Better data and better insights can then be taken forward, allowing sponsors to optimise future trials and, ultimately, the commercial launch.
RL CliniPilot works seamlessly with all our clinic-ready devices. All the devices share the same interface and the same colours, so they’re really easy for patients to use and for pharma partners to set up, including for blind studies. Let’s say that the viscosity of the drug and the placebo are different, so – to keep the trial truly blind – a different autoinjector can be used for each to keep the injection time similar. With our clinic-ready devices, that different autoinjector is already there, outwardly identical and ready to go.
Q Do you think that these approaches to clinical trials are carrying through to commercial products for regular patients?
RL In some ways, yes. For example, one of the advantages of our clinic-ready devices is that they can be used throughout the clinical studies, then the pharma partner can develop their own autoinjector for commercial use, with the full range of design options that our product portfolio provides.
“OVERALL, PHARMA PARTNERS CAN RELY ON ESTABLISHED AND PROVEN DOCUMENTATION FROM THE START OF DEVELOPMENT FOR AN EARLY CLINICAL STUDY UNTIL COMMERCIAL READINESS, WHICH INCREASES FAMILIARITY AND ENABLES A SMOOTH PROCESS AND COLLABORATION.”
There are several advantages for transitioning from the clinical to the commercial device. First, the user interface remains essentially the same. Second, the technical file of the clinical development can be reused and enriched for the commercial submission. Third, the drug injection profile is comparable, which allows the integration of the performance data from the clinical device into the documentation of the commercial device. Overall, pharma partners can rely on established and proven documentation from the start of development for an early clinical study until commercial readiness, which increases familiarity and enables a smooth process and collaboration.
Q Looking beyond clinical trials, what is the value of real-world injection data and patient-use insights in a commercial setting?
CC Real-world injection data and patient-use insights provide critical evidence for improving adherence, shaping patient support programs, and demonstrating value to payers and providers, ultimately driving better outcomes and brand differentiation in the commercial market, as already demonstrated across a few use cases.
SmartPilot, our commercial-stage solution, is FDA-cleared; we received 510(k) clearance in early September. CliniPilot, on the other hand, is designed for clinical trial use and does not require 510(k) clearance. Because both solutions function as add-on devices, pharmaceutical companies can integrate them during clinical development, and in the commercial setting across multiple therapeutic areas without impacting the regulatory approval process for the drug-device combination product.
Q From a broader perspective, how does this connected clinical development approach reflect Ypsomed’s long-term strategy in digital health and patient-centric innovation?
CC Our strategy is to scale connectivity on both the clinical development and the commercial side. Our goal is to enable pharma companies to collect more and better injection data to support clinical development and commercial launches. That’s what we’re focusing on for the near future. For the commercial setting, we’re looking at how best to integrate SmartPilot within digital health ecosystems, which in most cases involves companion apps, and how we can support pharma in differentiating their products across the entire product lifecycle.
Q Are there any final comments that you’d like to add before we wrap up?

Figure 4: YpsoMate autoinjector.
CC One extra thing I’d like to bring up regarding CliniPilot is that we’ve made it so that it can be integrated into existing clinical trial digital infrastructure. With multiple electronic data capture providers out there, we wanted to ensure that CliniPilot could easily be integrated into any solution that the trial’s pharma sponsor might already have in place. We’re committed to providing integration without disrupting the existing workflow.
RL There are already numerous successfully launched YpsoMate autoinjectors on the market (Figure 4). In addition, Ypsomed has demonstrated the performance of all its clinic-ready devices. The market-proven device, the performance data, as well as the support of Ypsomed device experts, help pharma partners to enter and derisk expensive early clinical studies with these devices.
Previous article
ACCELERATING PATIENT-CENTRIC THERAPIES FROM DEVELOPMENT TO DELIVERYNext article
TRENDS FOR 2026
