To Issue 134
Citation: Botha M, Bilstein A, Elghobary H, “A Game Changer for High-Risk Medications via Intranasal Drug Delivery Platform”. ONdrugDelivery, Issue 134 (Jun 2022), pp 38–34.
Marcel Botha, Andreas Bilstein, Houssam Elghobary and Rouven Kraus discuss the benefits of the Validose digital health platform for the safe delivery of high-value and/or high-risk products.
The tide is finally turning when it comes to covid-19 – and the world is ready to return to normal. Nevertheless, normal will mean facing several healthcare challenges that have been hiding in the shadow of the pandemic. The good news is that next-generation, evidence-based treatments exist for some of these insolvable dilemmas, such as widespread substance abuse, depression and chronic pain. The bad news is that those treatments include highly regulated drugs that have the potential to be abused.
According to the US Centers for Disease Control and Prevention’s (CDC) National Vital Statistics System, 103,598 Americans died from prescription opioid misuse in 2021.1 Every day, 46 people in the US die from overdosing on an opioid that was prescribed by a doctor. Of those who use heroin and other illegal opioids, 86% say they were put on that road by a legal opioid prescription for themselves or someone they knew.2 Prescriptions do not always go where they are supposed to when they leave the pharmacy – diversion is the black side of the opioid dilemma. And the majority of people who abuse these medications get them from family or friends.
During the covid-19 pandemic, the prevalence of moderate-to-severe depression in the US increased more than threefold from 8.5% before covid-19 to 27.8% during covid-19 (Figure 1).3 The estimated number of unreported cases is even worse.
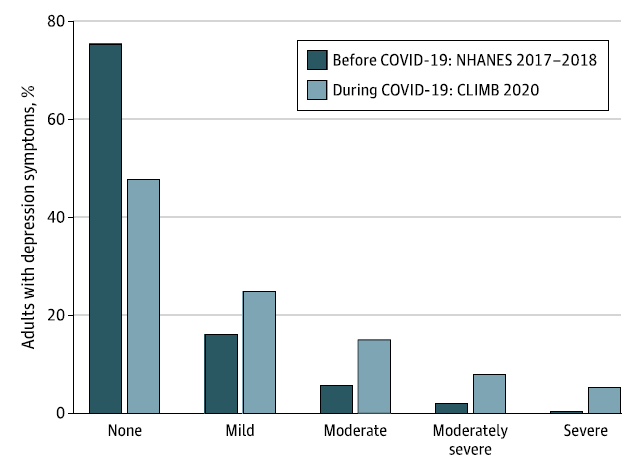
Figure 1: Depression symptoms in US adults before and during the covid-19 pandemic.
“The Validose system delivers scheduled medications based on an integrated but separately filled pump system.”
Coming to the second crisis, the psychiatric community is on high alert, and more promising medications are being developed now than in previous decades. Ketamine, a tightly controlled substance that has traditionally been used as a form of anaesthesia, has been shown to have transformative potential in the treatment of severe depression. Patients with depressive disorders, particularly treatment-resistant depression, have been found to benefit from ketamine nasal spray. It is quick acting, with clinical trials indicating that patients are relieved of suicidal ideation in as little as a few hours, and its effects can last up to a week. In comparison, most oral antidepressants can take weeks to start working.
These two crises have had a significant impact on Americans’ life expectancy. Safely delivering opioids and antidepressants could reverse these trends permanently, offering a high quality of life for those patients. This ongoing dilemma of how healthcare providers administer and monitor controlled substances triggered the invention of a device to solve these problems. And this was the spark to generate an innovative digital solution to address many of the trickiest modern problems in patient care, including the misuse of prescription medicines, the monitoring of dosage/adherence and, most importantly, the ability to improve patients’ lives.
Approximately 235 million prescriptions for painkillers, antidepressants and substance abuse treatments are written each year. Fentanyl and buprenorphine, for example, are high-risk medications. All these high-risk medications with lifesaving potential could potentially be used with a device offering advanced drug intake control and biometric features.4 Such a device has been presented under the name of Validose, and it has been sketched out to offer a solution that brings the future of safer medications through the nasal route of administration in which every dose is validated.
“One of the crucial considerations during the development of nasal dosage applications is to offer the possibility of avoiding preservatives, wherever possible.”
TEAM UP FOR AN INTEGRATED SUPPLY CHAIN
The Validose system delivers scheduled medications based on an integrated but separately filled pump system. It can only be activated by the patient’s fingerprint, which is detected by a sensor like that found on a smartphone. Fingerprint security is used to lock every single dose – ensuring that the right dose is delivered to the right person at the right time. Data about the dose is immediately shared with the physician’s office. A set of security measures built into the design of both the hardware and software prevents patients taking extra doses or sharing one with a friend and, if the patient misses a dose, the device can alert the user, prompting him to check-in. It is a simple idea that could transform the delivery technology for a whole spectrum of potentially serious medications (Figure 2).

Figure 2: The current Validose device design.
But the Validose device is only one part of the story. It needs to integrate with a precise and high-quality dispersing pump. Through a synergetic partnership between Validose, as the digital augmentation device designer, Aero Pump, as one of the world’s leading manufacturers specialising in the production of pharmaceutical spray pumps and droppers with high dosing precision, and URSATEC as the inventor of preservative-free multidose applications, an integrated device including nasal spray pump system, bottle, filling and integration into the digital augmentation was envisioned, to allow a fast and easy transfer of existing products or an optimal development of a new product.
THE RIGHT PUMP SYSTEM IN THE RIGHT DEVICE
The combined approach of Validose, Aero Pump and URSATEC allows optimisation of existing products by the use of precise metering pumps for preserved products from Aero Pump, with a toolbox of adjustments to fit the device to the existing product and the opportunity to either develop new products or switch the current product to a preservative-free application to enhance patients’ application experience. One of the crucial considerations during the development of nasal dosage applications is to offer the possibility of avoiding preservatives, wherever possible, as per the majority of global healthcare authorities’ recommendations.5 That is why the patented 3K®-nasal dosage system and the COMFORT® airless systems were invented. The 3k® system is a “non-airless” dosing pump that is used for the delivery of liquid pharmaceutical and medical preparations with triple protection technology against microbial contamination (Figure 3):
- Coil with a bacteriostatic surface
- Microbial tightly closing valve
- Air-purifying filter.
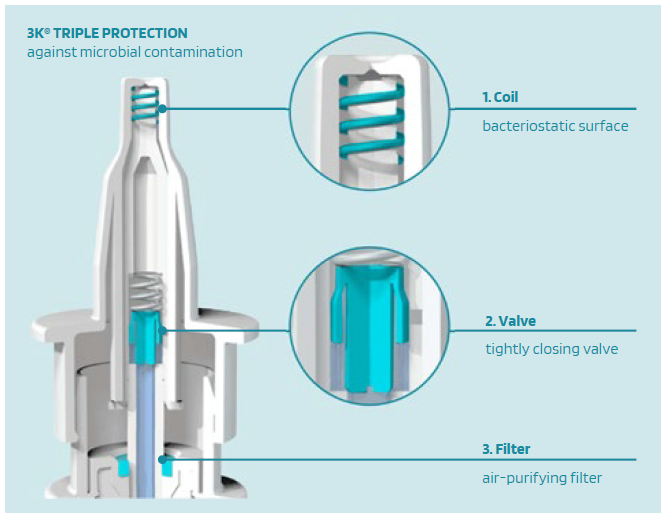
Figure 3: The triple protection technology of the 3k® pump.
THE COMBINED APPROACH/THE RIGHT SYNERGY
A complete hardware approach from the primary packaging system, including device-specific modifications, to the filling and assembly of the product and the digital augmentation of the container closure system, has been achieved by the collaboration between technology provider leaders to create high value for an integrated value chain of innovative personalised precision medicines.
The smart “Internet of Things” (IoT) exoskeleton that enables digital locking and unlocking is augmented to integrate with either Aero Pump’s pump portfolio or URSATEC’s patented triple-protection 3k® or COMFORT dosage pumps. For example, the specific modified and completely pre-assembled and sterilised 3k® pump is snapped on the sterilised primary container after the filling process, which is carried out under aseptic conditions to offer maximum protection against microbial contamination even for long-term safe use without the need for any preservatives (Figure 4). Another option would be Aero Pump’s spray-nozzle-unit-based nasal spray for optimised deposition for nose-to-brain delivery. In any case, the exoskeleton itself is reusable and can be used over a long period, whereas the nasal-spray system itself can be exchanged regularly. Even a leasing model with the patient and/or medication change could be realised with one exoskeleton.
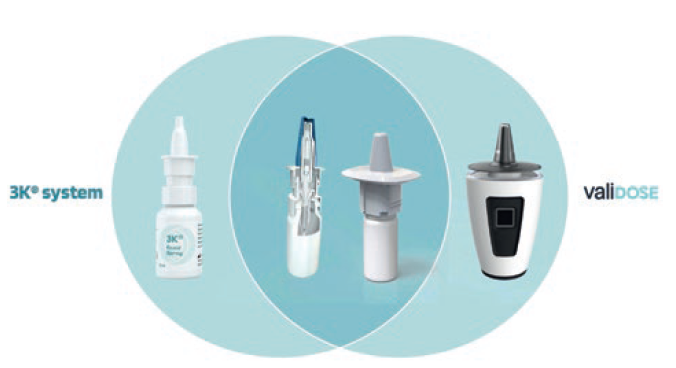
Figure 4: The synergistic approach of pump device and digital augmentation.
The exoskeleton is controlled by the Validose app. The app is focused on enabling the user to participate in real time. It drives adherence through schedule and dosage reminders to the phone and other digital peripherals such as smartwatches. Other stakeholders like doctors, caregivers and elected family members will have different app experiences derived from the same macro data set. Validose can automatically adjust for dynamic dosing protocols if the dose has to fluctuate during prescribed therapy. It records all aspects of delivered dose history, delivery compliance and dose adherence and gives accurate remaining dose information. The app receives both real-time and asynchronous data from the device. Figure 5 shows how simple the app is. It allows the user to have full data access to know the percentage of adherence, dose history, doses remaining and medication types.
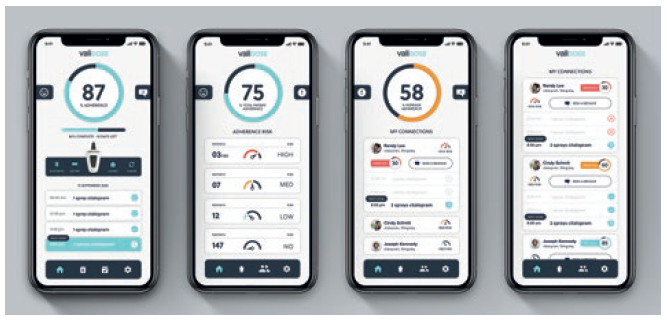
Figure 5: The Validose software is simple and user friendly.
To complete the ecosystem, a cloud-based patient management platform was established (Figure 6), which aims to give physicians real-time insight into large patient populations such as indication interactions, medication type or concentration, patient adherence and prescription tracking. The platform aggregates patient data over time and at scale, giving the provider’s office granular monitoring of patient-care quality and outcome over the entire course of their treatment. The quantitative data can then be matched with qualitative feedback from caregivers, providers and patients themselves to get a holistic picture of the patient’s perceived and observed progress.
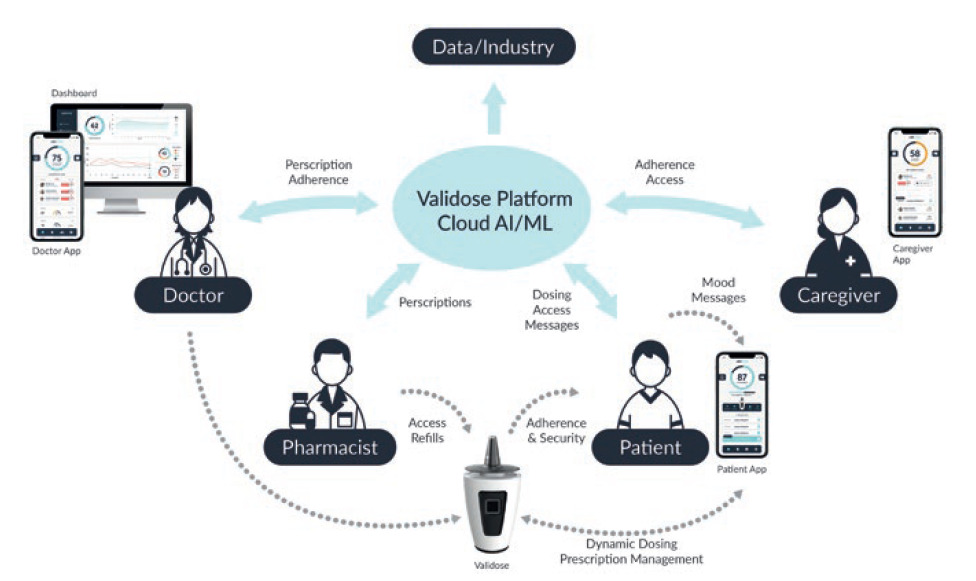
Figure 6: A complete ecosystem and cloud-based patient management platform overview.
“The Validose digital health platform offers the safe delivery
of high-value and/or high-risk products while providing
long-term adherence solutions for all engaged stakeholders.”
WHAT IS IN IT FOR THE ENGAGED STAKEHOLDERS?
The patient:
- One device, one user, no diversion – a pocket-sized device provides only one patient with a secure and reliable way to get high-risk medications. If the user unlocks his dose and hands it over to a second user, motion-sensing technology immediately locks the device.
- Unlock to have your dose – a dose is only delivered when a patient places their fingerprint on a small sensor; it prevents accidental double dosing while verifying every dose taken.
- An integrated cloud ecosystem – the device sends a tracking report digitally when a dose is taken, including the time and location of the treatment, keeping both the patient and the prescriber informed.
- Tamper-resistant design – the patient can only get his prescribed dose and then the device is locked. A mechanical security feature is triggered if someone tries to force open the device. If the device is intentionally crushed, the internal glass bottle will break, allowing all medication to leak out and be neutralised by an absorption layer in the surrounding chamber.
- A long-life solution – a pharmacist can easily refill the device. Patients would get access to a smartphone app that tells them when their refills are due, how their medication usage is tracking over time and when their next appointment with the physician is.
- The combination of a highly precise nasal-spray system with a preservative-free product.
The provider:
- A unique combination of application technology, digital augmentation and product realisation capacities which allows the transfer or development and manufacture of all kinds of nasal-spray products.
- Unbiased care – cutting-edge treatments like ketamine are often only accessible to a selected group of people who reach a restricted audience. These high-risk medications can be distributed more widely and equitably if providers have more control.
- Case tracking and adherence:
– Pharmaceutical companies have a dashboard that shows when the device is used, making patient communication more efficient, especially during the clinical trials phase.
– Clinicians can also track patient usage and adherence habits, allowing them to follow up if a dose is missed. - Cost savings:
– Patients avoid unnecessary visits to the doctor’s office to receive their high-risk medications that need to be taken on-site.
– Better adherence with a lower risk of abuse reflects significant cost effectiveness and improved patient health outcomes. - Medico-legal support:
– Security features prevent misuse by healthcare professionals.
– Any professional misconduct attempt to order a refill from the pharmacy, for example, will lock out the provider and trigger emergency alarms in the reporting system.
CONCLUSION
There is a current, and accelerated, need for novel at-home continuity of care solutions. Beyond remote consultations and monitoring, there is a need for self administration and better adherence to high-risk drugs. The Validose digital health platform offers the safe delivery of high-value and/or high-risk products while providing long-term adherence solutions for all engaged stakeholders – for example, patients, physicians and payers.
Validose provides a standardised augmentation strategy by digitally enabling the application devices from Aero Pump and URSATEC. The existing medication packaging supply chains can be augmented to integrate with the smart IoT exoskeleton, and no change in function is required for the existing packaging manufacturer or supplier.
Custom-developed drugs or new drug launches can directly use the combined development services for the digitised product line available. Aero Pump and URSATEC offer development expertise for primary packaging, high-tech medical devices and dosage systems, as well as pharmaceutical formulations, to create new business. Collectively, the partners of the combined approach have in-depth expert knowledge of development and registration to enable quick market entry and guarantee compliance with national and international guidelines.
Finally, there is no end in sight for the telehealth and remote care changes that the pandemic brought about. What felt new and early to market in 2019 has become mission critical for continuity of care in 2022. In the US, Europe and beyond, we will see continued investment expansion and integration of digital health services with the intent of improving access, reducing risk and, ultimately, enhancing patient outcomes.
REFERENCES
- “Provisional Drug Overdose Death Counts”. CDC, accessed May 19, 2022.
- Baumgartner J, Radley D, 2021. “The Spike in Drug Overdose Deaths During the COVID-19 Pandemic and Policy Options to Move Forward”. The Commonwealth Fund, Mar 25, 2021.
- Ettman C et al, “Prevalence of Depression Symptoms in US Adults Before and During the COVID-19 Pandemic”. JAMA Network Open, 2020, Vol 3(9), pp 1–10.
- “A Game Changer for High-Risk Medications”. Health, 2021.
- Barnes K, 2006. “Aseptic nasal spray manufacturing an untapped market”. Outsourcing-pharma.com, Jun 20, 2006.

