To Issue 157
Citation: Daull P, Roy P, “Accurate Delivery and Precise Dosing of Eye Drops: What if We Change the Instillation Procedure?”. ONdrugDelivery, Issue 157 (Mar 2024), pp 4–9.
Philippe Daull and Pierre Roy discuss how changing the administration procedure for eye drops can improve the patient experience and eliminate health risks.
“Received wisdom confirms that the accurate delivery of a single, well-calibrated drop remains a challenge for a very large proportion of patients, especially for elderly or visually impaired patients.”
Topical ocular administration of drugs is a critical component of the treatment of ocular diseases, such as glaucoma, dry eye disease, Sjögren’s syndrome and allergies, to name a few. Currently, the most common way to administer treatment to the eye is through the administration of an eye drop via the use of a multidose (MD) eyedropper. The eye drop delivers the medication directly to the affected area, providing local and immediate relief.
For the past 70+ years, administering an eye drop required the patient to tilt their head backwards, raise their arm above their head and, in this uncomfortable position, perform the delicate operation of targeting the eye and controlling the squeezing force to expel a single drop from the MD eyedropper, and all without a clear visual of what they are doing. Administering an eye drop is neither straightforward or easy.
Received wisdom confirms that the accurate delivery of a single, well -calibrated drop remains a challenge for a very large proportion of patients, especially for elderly or visually impaired patients. The most frequent problems encountered are difficulty targeting the eye (up to 76% miss the eye completely), uncontrolled number of drops expelled upon squeezing (up to 64% of the patients dispense more than one drop) and frequent inadvertent contact between the tip of the MD eyedropper and the eye structures (studies show that the tips have been contaminated in almost 80% of patients’ MD eyedroppers).1,2 Table 1, adapted from Hovanesian et al, summarises the main issues associated with the instillation of eye drops and their related risks.1
| Issues with eye drop administration | Frequency | Risks |
| Overall failure rate19–23 | 13%–91% | Non-adherence, disease progression and vision loss |
| Contamination of eyedropper tip through contact with the eye or lid19–22,24–27 |
18%–76% | Eye infection Cornea trauma following inadvertent contact with the tip |
| Missing the eye20,21,24,25 | 10%–76% | Disease progression Multiple attempts, product spillage |
| Difficulty aiming28 | 49% | Periocular side effects Multiple attempts, product spillage |
| Dispensing more than one drop20,21,27,29,30 | 11%–64% | Side effects (ocular and periocular) Product spillage, increase cost for treatment, over-prescription |
| Difficulty squeezing28 | 20% | Stop treatment, disease progression |
Table 1: Common issues with the instillation of eye drop medications with existing MD eyedroppers and their associated risks.
The difficulty with administering eye drop medication is the root cause of significant health risks for patients, including poor compliance and treatment cessation, leading to disease progression, poor quality of life and vision loss.3–6 Note that forgetfulness, complicated dosing regimens, ocular or periocular side effects (due to overdosing) and the cost of eye drop medication are also associated with the poor compliance observed in patients with chronic eye diseases, such as glaucoma and Sjögren’s syndrome.
Eye infection, resulting from tip contamination of MD eyedroppers, has often been identified as a potential risk for ocular health. However, even though the majority of MD eyedropper tips are contaminated following inadvertent contact between the tip and the eye structures (cornea and conjunctiva) or the eyelids and eyelashes (up to 80%, see Table 1), or simply from the environment, the US FDA’s Ophthalmic Devices Panel of the Medical Device Advisory Committee (MDAC) states: “It may be concluded that the ophthalmic dispensers are generally low in risk”.7
“The administration procedure itself is at the heart of the issues and risks associated with MD eyedroppers.”
The healthy microbiome naturally present on the ocular surface, which is closely related to the microbiome of the eyelids, has been demonstrated to protect the eye from pathogenic infection.8–11 Indeed, the low prevalence of eye infection observed over the past decades – despite the long history of use of MD eyedroppers with tips contaminated with patients’ own ocular/eyelid microbiome – explains why the MDAC views MD eyedroppers as “low in risk”. This must be distinguished from the recent warning letters issued by the FDA for a potential risk of eye infection following the use of eye drops and recalls that were related to unsanitary conditions and sterility breaches of critical drug production areas at the manufacturing facility, with the subsequent possible contamination of the incriminated MD eyedroppers’ content during the manufacture of the drug product.12–14
It is very important that eye drop formulations are manufactured under sterile conditions and are adequately protected, either with effective preservative agents or preservative-free MD eyedroppers for the safe use of the eye drop medication.15
The difficulties of administering eye drops with existing MD eyedroppers led Menino et al to state that “new strategies must be developed, such as creating new containers that are easier to handle for the elderly”.16 This also suggests that existing MD eyedroppers are ineffective drug delivery devices, as they fail to accurately deliver a single, well-calibrated eye drop (Table 1), posing serious risks to patients’ eye health and vision.
The administration procedure itself is at the heart of the issues and risks associated with MD eyedroppers. Since the procedure is directly linked with the design of existing MD eyedroppers, is it feasible for this design to progress towards a new drug delivery device where the complicated administration procedure is replaced by an easier and safer application procedure (Figure 1) that does not require patients to recline their head backwards, for example? Could those design changes benefit patients’ health and quality of life?
A redesigned MD eyedropper should:
- Resolve the administration difficulties faced by patients when they try to administer an eye drop, such as by changing the administration procedure itself.
- Be easy to use, giving patients better control of the administration procedure and more confidence in the fact that the eye drop is accurately delivered to the eye with no product spillage, such as by giving patients the ability to see what they are doing while administering an eye drop (i.e. removing the need for patients to recline their head backwards).
- Have reasonable manufacturing costs to better manage the expense of eye drop medication.
A new MD eye bottle that can be used in an upright position – allowing the patient to keep their head straight in a comfortable manner, does not need them to raise their arm and allows them to see and control what they are doing (through the use of a mirror or a smartphone in selfie mode) – should improve the patient experience and satisfaction with eye drop medication. Figure 1 schematically illustrates this new concept, where the instillation procedure (where the eye drop falls on the eye) is replaced by an application procedure (where the eye drop is directly transferred from the drug delivery device to the conjunctival fold).
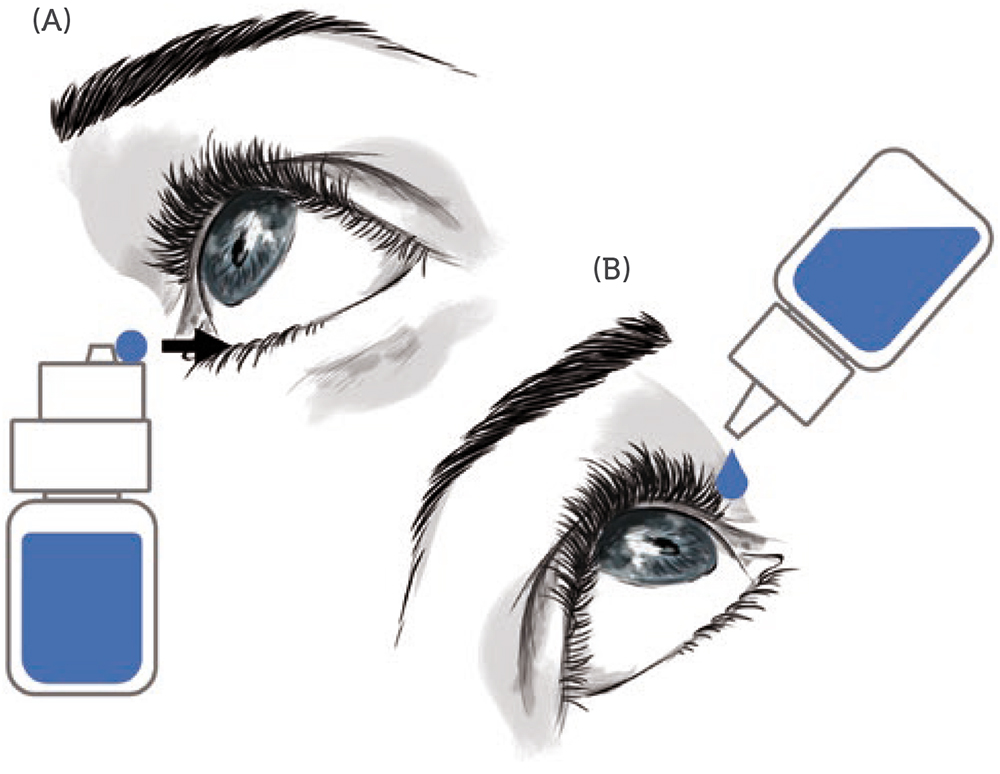
Figure 1: The application vs administration of eye drop medication. A) Application: the patient keeps their head straight, and upon opening of the conjunctival fold, the eye drop is transferred to the eye with the new MD eye bottle in an upright position. B) Instillation: the patient reclines their head backwards and let a drop fall into the eye, while the MD eyedropper is in an inverted position, raised above the head and pressed.
Table 2 highlights the key attributes that an MD eye bottle should possess to be a user-friendly, accurate and reliable drug delivery device.
| Desired quality | Specific attributes |
| Easy to use | • Use new MD eye bottle in an upright position • No tilted head or raised arm position • See and control all steps of the drop application process throughout the process • Secure and stable application gesture (use the cheekbone as a guide) • Easy to understand the application process and how to use/orientate the new MD eye bottle (“look and feel”) • Easy two-step application gesture – perform one action at a time (expel and apply the drop in a sequential manner) |
| Resolve the administration issues | • Accurately target the eye every time • Apply only a single eye drop • Well-calibrated eye drop (all drops of exactly the same volume – eye drop volume independent of the pinching pressure and bottle angle) • No product spillage • Eliminate the backwash of liquid (i.e. expelled liquid re-aspiration within the bottle) • Tip should not touch the eye (avoid cornea trauma, decrease contamination risk) • Head/nozzle delivery surface should be hydrophobic (to eliminate any residual liquid) • Easy to control the pinching pressure to expel a single eye drop (operation performed in a comfortable position – clear visual of the operation) |
| Affordable | • Simple design of the new MD eye bottle head/nozzle • Low manufacturing cost by injection moulding |
Table 2: Desired qualities for a new user-friendly MD eye bottle.
“Patients were particularly satisfied that the risk of product spillage is also reduced with the new MD eye bottle, and that the risk of touching the cornea is greatly reduced.”
Accurately applying a single, well calibrated eye drop into the conjunctival fold of the eye is possible with the new MD eye bottle. Figure 2 illustrates the two actuation and application steps for accurate administration of an eye drop. Note that the patient can, at all times, easily see and control what they are doing. For actuation, a simple up and down movement enables the patient to easily expel a single, well-calibrated drop and put it on the hydrophobic delivery surface by pressing on the MD eye bottle when it is returned to its upright vertical position. The volume of the drop is independent of the pinching pressure and is governed by the internal design of the bottle.
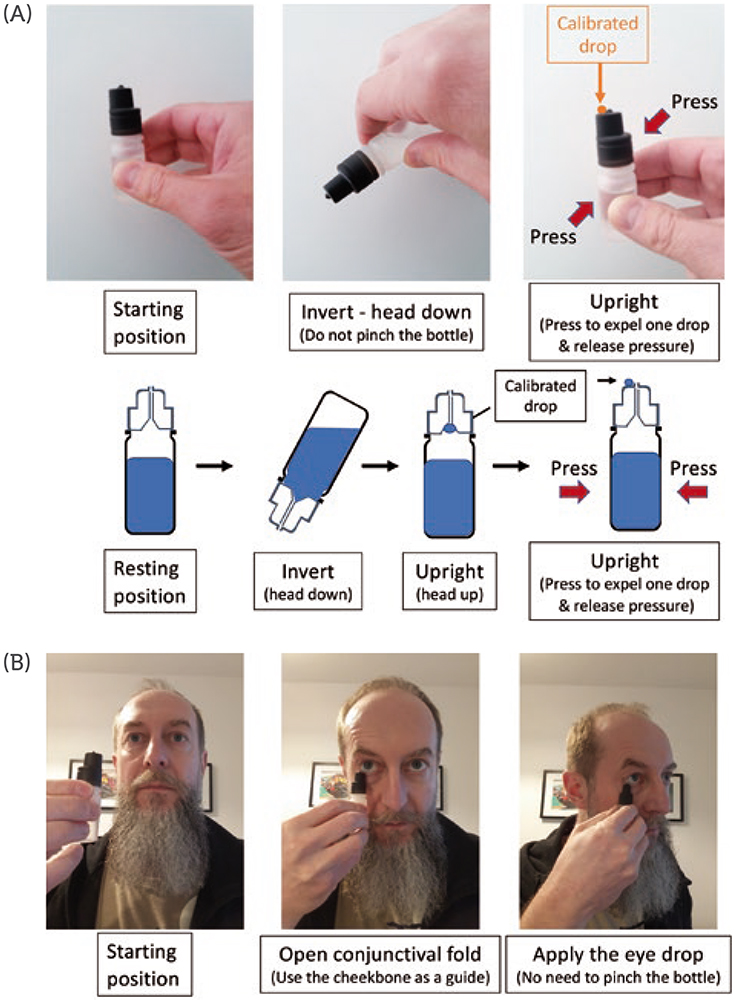
Figure 2: Schematic representation of the two-step application procedure of the new MD eye bottle. (A) actuation and (B) application steps of a single, well-calibrated eye drop with the new MD eye bottle.
The application requires the conjunctival fold to be gently opened with transient contact between the lower eyelid margin and the external rim of the new MD eye bottle. There is no need to touch the eyeball to transfer the drop (by capillary attraction) from the delivery surface to the tear film in the conjunctival cul-de-sac. In terms of eye infection risk, this application gesture is very close to the “closed eyelid instillation” recommended by the American Academy of Ophthalmology for children or people too anxious to administer an eye drop – the eye drop is deposited on the closed eyelid in the nasal corner of the eye and then rolls into the eye upon eyelid opening and blinking.17 With this procedure, the eye drop may be contaminated by bacteria on the eyelid as it rolls into the eye.
To assess patient perception and acceptance of this change in application, a preliminary usability study with the new MD eye bottle was performed.18 Sixteen patients (both naive and experienced with existing MD eyedroppers) were presented with the new MD eye bottle and the instruction leaflet. Following a two-minute demonstration of the correct use of the new MD eye bottle, patients were asked to test it. A questionnaire and a five point Likert scale survey evaluated their understanding of the use instructions and their appreciation of the new application procedure. Patient feedback on the strengths, weaknesses and advantages over the existing MD eyedroppers was also recorded.
“Frankly easier”, “Much more convenient than classic droppers”, “You can even use it with glasses on, this is positive, you can see what you do”, “Gesture more evident compared to when you need to raise the arm. More comfortable for the neck” and “It is convenient, and new. No spillage, you do not put liquid everywhere.”
A total of 15 out of 16 (93.8%) patients preferred the new MD eye bottle over existing MD eyedroppers. The new application gesture was rated as easy to perform, and the new MD eye bottle was either very easy (75.0%) or easy (18.8%) to use for 15 out of 16 patients (Figure 3). Patients were particularly satisfied that the risk of product spillage is also reduced with the new MD eye bottle, and that the risk of touching the cornea is greatly reduced. The feedback was very positive, with comments such as: “Frankly easier”, “Much more convenient than classic droppers”, “You can even use it with glasses on, this is positive, you can see what you do”, “Gesture more evident compared to when you need to raise the arm. More comfortable for the neck” and “It is convenient, and new. No spillage, you do not put liquid everywhere.”
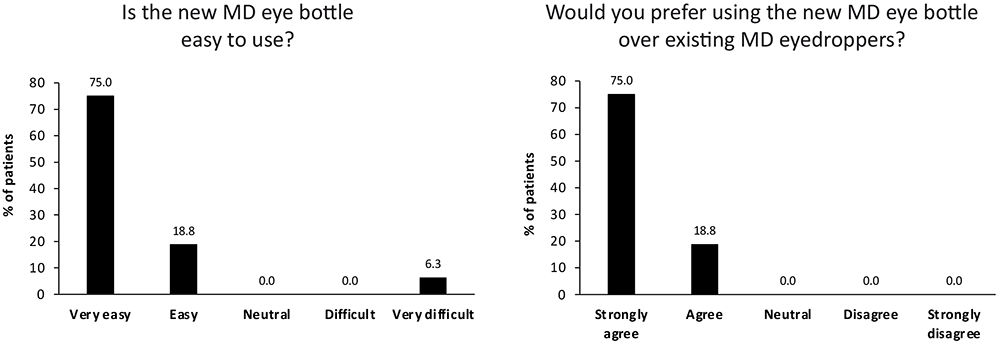
Figure 3. Usability testing results for new MD eye bottle.
This usability study determined that this patient-centric design for the new MD eye bottle is easy to use and the new application procedure is well accepted. This new design improves patients’ accuracy and ability to correctly deliver the right dose of treatment while reducing product spillage. By improving the topical ocular administration experience and satisfaction, the new design may help improve treatment adherence. The new MD eye bottle has the potential to better protect patients’ vision and improve their quality of life.
In conclusion, it is possible to change the way eye drops are dispensed through a simple evolution of the design of existing MD eyedroppers. The benefits the new application gesture can bring to patients is clear, and patients are keen to change from existing MD eyedroppers – which are far too complicated to use – to the more user-friendly design of the new MD eye bottle. Importantly, the new design does not create any new risks for patients and uses proven technologies compatible with regulatory and economic requirements.
REFERENCES
- Hovanesian J et al, “Identifying and addressing common contributors to nonadherence with ophthalmic medical therapy”. Curr Opin Ophthalmol, 2023, Vol 34 (Suppl 1), pp S1–S13.
- Radcliffe NM, Shah M, Samuelson TW. “Challenging the “Topical Medications-First” Approach to Glaucoma: A Treatment Paradigm in Evolution”. Ophthalmol Ther, 2023, Vol 12(6), pp 2823–2839.
- Singh K et al, “Factors affecting adherence to glaucoma medication: Patient perspective from North India”. Indian J Ophthalmol, 2023, Online ahead of print.
- Tanito M et al, “Factors Associated with Topical Medication Instillation Failure in Glaucoma: VRAMS-QPiG Study”. Adv Ther, 2023, Vol 40(11), pp 4907–4918.
- Michaelov E et al, “Sjögren’s Syndrome Associated Dry Eye: Impact on Daily Living and Adherence to Therapy”. J Clin Med, 2022, Vol 11(10), p 2809.
- Gupta D et al, “Cost-Related Medication Nonadherence in a Nationally Representative US Population with Self-Reported Glaucoma”. Ophthalmol Glaucoma, 2021, Vol 4(2), pp 126–130.
- “November 10, 2022: Ophthalmic Devices Panel of the Medical Devices Advisory Committee Meeting Announcement”. US FDA, Nov 10, 2022.
- Spencer SKR, Francis IC, Coroneo MT, “Spontaneous face- and eye-touching: Infection risk versus potential microbiome gain”. Ocul Surf, 2021, Vol 21, pp 64–65.
- Kugadas A et al, “Impact of Microbiota on Resistance to Ocular Pseudomonas aeruginosa-Induced Keratitis”. PLoS Pathog, 2016, Vol 12(9), Article 1005855.
- Garza A et al, “Homeostasis and Defense at the Surface of the Eye. The Conjunctival Microbiota”. Curr Eye Res, 2021, Vol 46(1), pp 1–6.
- Ren Z et al, “Profiling of the Conjunctival Bacterial Microbiota Reveals the Feasibility of Utilizing a Microbiome-Based Machine Learning Model to Differentially Diagnose Microbial Keratitis and the Core Components of the Conjunctival Bacterial Interaction Network”. Front Cell Infect Microbiol, 2022, Vol 12, Article 860370.
- Mughal S, Sakina SK, “Artificial tears: Promising treatment or silent threat to public health?”. Health Sci Rep, 2023, Vol 6(8), Article 1508.
- “Outbreak of Extensively Drug-Resistant Pseudomonas Aeruginosa Associated with Artificial Tears”. Centers for Disease Control and Prevention, Accessed Feb 2024.
- “FDA Warns Consumers Not to Purchase or Use EzriCare Artificial Tears Due to Potential Contamination”. US FDA, Accessed Feb 2024.
- Campolo A, Crary M, Shannon P, “A Review of the Containers Available for Multi-Dose Preservative-Free Eye Drops”. BJSTR 2022, Vol 45(1), pp 36035–36044.
- Menino J, Camacho P, Coelho A, “Initial medication adherence in newly diagnosed glaucoma patients: three adherence measures”. Int J Ophthalmol, 2023, Vol 16(4), pp 630–637.
- Gudgel DT, “How to Put in Eye Drops”. American Academy of Ophthalmology, May 5, 2023, available from: https://www.aao.org/eye-health/treatments/how-to-put-in-eye-drops, accessed Feb 2024.
- Roy P, Daull PA, “New Eye Drop Delivery Device That Resolves Patient’s Ocular Instillation Issues”. Poster Presentation, ARVO, New Orleans, 2023.
- Usgaonkar U, Zambaulicar V, Shetty A, “Subjective and objective assessment of the eye drop instillation technique: A hospital-based cross-sectional study”. Indian J Ophthalmol, 2021, Vol 69(10), pp 2638–2642.
- Gomes BF et al, “Assessment of eye drop instillation technique in glaucoma patients”. Arq Bras Oftalmol, 2017, Vol 80(4), pp 238–241.
- Kashiwagi K et al, “Investigation of visual and physical factors associated with inadequate instillation of eyedrops among patients with glaucoma”. PLoS One, 2021, Vol 16(5), Article e0251699.
- Gupta R et al, “Evaluating eye drop instillation technique in glaucoma patients”. J Glaucoma, 2012, Vol 21(3), pp 189–192.
- Stone JL et al, “An objective evaluation of eyedrop instillation in patients with glaucoma”. Arch Ophthalmol, 2009, Vol 127(6), pp 732–736.
- Tatham AJ et al, “Eye drop instillation technique in patients with glaucoma”. Eye (Lond), 2013, Vol27(11), pp 1293–1298.
- Schwartz GF, Hollander DA, Williams JM, “Evaluation of eye drop administration technique in patients with glaucoma or ocular hypertension”. Curr Med Res Opin, 2013, Vol 29(11), pp 1515–1522.
- Naito T et al, “Comparison of success rates in eye drop instillation between sitting position and supine position”. PLoS One 2018, Vol 13(9), Article e0204363.
- Lazcano-Gomez G et al, “Videographic Assessment of Glaucoma Drop Instillation”. J Curr Glaucoma Pract, 2015, Vol 9(2), pp 47–50.
- Winfield AJ et al, “A study of the causes of non-compliance by patients prescribed eyedrops”. Br J Ophthalmol, 1990, Vol 74(8), pp 477–480.
- An JA et al, “Evaluation of eyedrop administration by inexperienced patients after cataract surgery”. J Cataract Refract Surg, 2014, Vol 40(11), pp 1857–1861.
- Liu Y et al, “Proficiency of eye drop instillation in postoperative cataract patients in Ghana”. Clin Ophthalmol, 2013, Vol 7, pp 2099–2105.
Previous article
HOW DIABESITY IS SHAPING THE WORLD OF SELF-INJECTION DEVICESNext article
LISTENING TO THE PATIENT’S VOICE TO IMPROVE EYE CARE
