To Issue 179
Citation: O’Neill C, Paterson D, Adams M, “Adoption of Sustainability Practices Within Subcutaneous Drug Delivery: Industry Insights”. ONdrugDelivery, Issue 179 (Oct/Nov 2025), pp 20–26.
Conor O’Neill (GSK), Duncan Paterson (AstraZeneca) and Dr Monica Adams (GSK) come together to discuss a recent study conducted by the Subcutaneous Drug Development & Delivery Consortium among its member organisations, digging into the pharmaceutical industry’s progress and priorities when it comes to improving the environmental impact of subcutaneous medicines.
The pharmaceutical industry is making substantial efforts to reduce its environmental impact and contribute towards global climate goals. Most pharma companies are aligned with international initiatives such as the Paris Agreement and the United Nations’ “Race to Zero” campaign, and have made public commitments to reduce carbon emissions across the entire value chain by 2045, with interim targets set between 2025 and 2030 to ensure progress, according to a survey of various company websites.
While there is a clear drive for change, realising pharma companies’ ambitions will require greater collaboration and industry alignment towards implementation of sustainability efforts across the subcutaneous (SC) product value chain, from raw materials through to end use. Currently, environmentally oriented solutions are limited and often only occur at the local product or company level, thereby minimising the opportunities available and their effectiveness.
“THE OBJECTIVES OF THE STUDY WERE TO INFORM FUTURE INITIATIVES OF THE SUSTAINABILITY SUB-TEAM, AS WELL AS TO IDENTIFY OPPORTUNITIES FOR BROADER COLLABORATION ON ENVIRONMENTAL SUSTAINABILITY EFFORTS ACROSS THE BIOPHARMA AND PHARMACEUTICAL INDUSTRIES.”
Against this backdrop, the Sustainability Sub-Team of the Subcutaneous Drug Development & Delivery Consortium (SC Consortium) conducted a study of its member organisations to benchmark environmental sustainability-related commitments, ambitions and perspectives across the industry. The objectives of the study were to inform future initiatives of the Sustainability Sub-Team, as well as to identify opportunities for broader collaboration on environmental sustainability efforts across the biopharma and pharmaceutical industries.
The study was conducted in two phases. The first was an online quantitative pre-work questionnaire completed by representatives from each member organisation (with the support of subject matter experts within their organisations as needed). The second phase involved in-depth, qualitative, discussion-based interviews with the respondents of the first phase together with other expert representatives of each participating company.
The discussion was designed to collect qualitative, contextual information about the quantitative ratings provided in the first phase and more generally about adoption and implementation of sustainability improvement practices within the member companies. Responses from individual interview participants were then reviewed and consolidated to achieve industry insights. While the majority of respondents were employed in packaging or device development functions within their companies, experts from environmental sustainability, drug development, commercial and regulatory affairs were also included. Companies varied with regard to the number of individuals providing responses (Table 1).
| SC Consortium Member Organisations (N=12) | Survey Respondents (N=12) | Interview Participants (N=29) |
| AstraZeneca | 1 | 3 |
| BD | 1 | 3 |
| Biogen | 1 | 1 |
| Boehringer Ingelheim | 1 | 2 |
| Bristol Myers Squibb | 1 | 3 |
| GlaxoSmithKline | 1 | 3 |
| Halozyme | 1 | 1 |
| J&J Innovative Medicine | 1 | 4 |
| Merck | 1 | 3 |
| Novartis | 1 | 3 |
| Pfizer | 1 | 1 |
| Sanofi | 1 | 2 |
Table 1: Number of representatives for each SC Consortium member organisation participating in the sustainability benchmarking study.
COMMON THEMES ABOUT SUSTAINABILITY COMMITMENTs AMONG MEMBER ORGANISATIONS

Figure 1: Existing commitments of Consortium member organisations towards future implementation of sustainable practices in product development.
Strong, corporate-level sustainability commitments aimed at achieving major reductions in environmental impact have been made across the SC Consortium’s member organisations. However, the study results indicate that, despite this corporate-level commitment, the transition to less environmentally impactful practices is lagging across all participating companies (Figure 1).
“QUALITATIVE DISCUSSIONS SUGGESTED THAT, TO DATE, MOST MEMBER COMPANIES HAVE CONCENTRATED THEIR SUSTAINABILITY EFFORTS ON AREAS THAT ARE COMPARATIVELY EASIER TO ADDRESS, SUCH AS SECONDARY AND TERTIARY PACKAGING.”
Qualitative discussions suggested that, to date, most member companies have concentrated their sustainability efforts on areas that are comparatively easier to address, such as secondary and tertiary packaging. In contrast, more complex domains – such as drug product formulation, device redesign and supply chain transformation – remain less advanced. A second theme concerned the regulatory environment, which participants described as both a challenge and an enabler; regulations can create prohibitive burdens for implementation, yet they are also viewed as essential for establishing a level playing field and compelling the industry to adopt more difficult but necessary changes.
Companies have identified that packaging is an area where environmental improvements can be implemented with lower complexity and risk compared with the drug product or manufacturing processes (Table 2), which can serve as an enabler for broader sustainability transitions. Furthermore, respondents scored packaging transitions highest in terms of the degree to which they were on track with corporate sustainability goals and targets.
| Product Development Process | Degree to Which Sustainable Transitions Are On Track* |
| Product Development Overall | 5.6 |
| Packaging | 5.8 |
| Procurement | 5.5 |
| Supply Chain | 5.3 |
| Product Development – Devices | 5.2 |
| Manufacturing – Devices | 5.1 |
| Product Development – Formulation | 4.7 |
| Manufacturing – Formulation | 4.7 |
Table 2: Degree to which Consortium member organisations self-assess being on track with future transitions towards more sustainable practices. *Average score on scale from 1 (not at all on track) to 7 (completely on track).
Table 3 highlights that secondary and tertiary packaging are the areas where green credentials are most likely to be considered compared with other aspects of product development. Table 4 shows that energy consumption, supplier selection and waste reduction are the key factors influencing selection decisions during development and process selection.
| Product Development Process | Degree to Which Green Credentials Are Considered* |
| Secondary/Tertiary Packaging | 5.2 |
| Devices – Product Design | 4.3 |
| Primary Packaging | 4.1 |
| Devices – Manufacturing Processes | 3.9 |
| Drug Product – Formulation Development | 3.9 |
| Drug Product – Manufacturing Processes | 3.9 |
Table 3: Degree to which green credentials are considered in Consortium members’ product development processes. *Average score on scale from 1 (not at all considered) to 7 (significant consideration).
| Green Credential Selection Factors | Importance When Selecting Green Credentials* |
| Energy Consumption | 5.5 |
| Supplier Selection | 5.5 |
| Waste Reduction | 5.3 |
| Device Material Selection | 5.1 |
| Container Selection/Primary Packaging | 4.8 |
| Drug Product Formulation Ingredients | 4.4 |
| Selection of Excipients | 4.3 |
| Automation | 3.7 |
Table 4: Perceived importance of factors when selecting product development and manufacturing processes with green credentials. *Average score on scale from 1 (not important) to 7 (highly important).
CONFLICTING PRIORITIES IMPACT IMPLEMENTATION OF REUSABLE AND MULTIDOSE DEVICES DESPITE ACKNOWLEDGED ENVIRONMENTAL BENEFITS
The study confirmed that the environmental benefits of reusable and multidose SC delivery devices over single-use devices are widely recognised because of the significant reduction in material use, waste and emissions per dose. However, despite these compelling benefits, there is not yet widespread adoption of these more complex devices, especially of those that require additional training or operational steps. Study responses confirmed that Consortium member organisations are hesitant to adopt these technologies because of the possible use-related risks they are perceived to bring. Furthermore, when considering reusable devices, factors such as the intervals between doses and use-risks – such as “memory-decay” known to occur after training with more complex systems – are barriers to adoption.
ALTERNATIVE MATERIALS AND PROCESS APPROACHES TO REDUCE ENVIRONMENTAL IMPACT
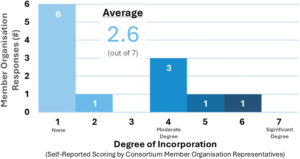
Figure 2: Current incorporation of non-fossil-derived or recycled polymers in Consortium members’ SC delivery devices.
The study indicates that alternative approaches to achieving sustainability goals are seen as easier to adopt, such as adjustments to material selection and sources of energy for the production of glass, plastic and metal components. Alternative plastics, for example, bio-sourced resins, can be used for packaging and device components to reduce environmental impact; however, to date, member organisations report limited incorporation of non-fossil-derived or recycled polymers in their products (Figure 2). These changes can be challenging to implement, given the regulatory implications, stringent safety and quality requirements, and concerns related to ethical sourcing. However, study responses indicate that alternatives to petrochemical-derived resin materials are considered to be important for achieving sustainability goals (Table 5).
| Polymer Type | Importance for Achieving Sustainability Goals* |
| Bio-based Polymers | 5.2 |
| Virgin Petrochemically-Derived Polymers | 5.2 |
| Biomass Polymers | 4.5 |
| Chemically Recycled Polymers | 3.8 |
| Carbon-Captured Polymers | 3.7 |
| Mechanically Recycled Polymers | 3.0 |
Table 5: Importance of plastic material types for achieving Consortium members’ sustainability goals. *Average score on scale from 1 (not important) to 7 (highly important).
Lower scores for the importance of certain available polymer options may be down to a lack of familiarity or experience with these newer materials or feedstock flows. Moreover, qualitative responses indicated that companies remain cautious due to supplier dependency and uncertainties related to quality and consistency, as well as broader environmental impacts, including biodiversity and land use.
The qualitative responses from the second phase of the study indicate that companies consider the ethical concerns that accompany bio-derived feedstock sources for bio-based plastic resin production, specifically including risks related to the displacement of agricultural land at the expense of food production and of impacts to rainforests. Nevertheless, there is some expressed interest from companies in the introduction of lower-impact plastics through alternative hydrocarbon sources or mechanical recyclate. Provided that outstanding concerns related to consistent material quality control, relative availability, cost and true environmental benefit can be addressed, we may start to see a reduced reliance on non-renewable resources in the industry.
Circularity
Consortium member organisations are conscious of the loss of high-value materials contained within injection devices upon disposal. However, most struggle to translate circularity ambitions into real action to prevent loss to landfill or incineration (Figure 3).
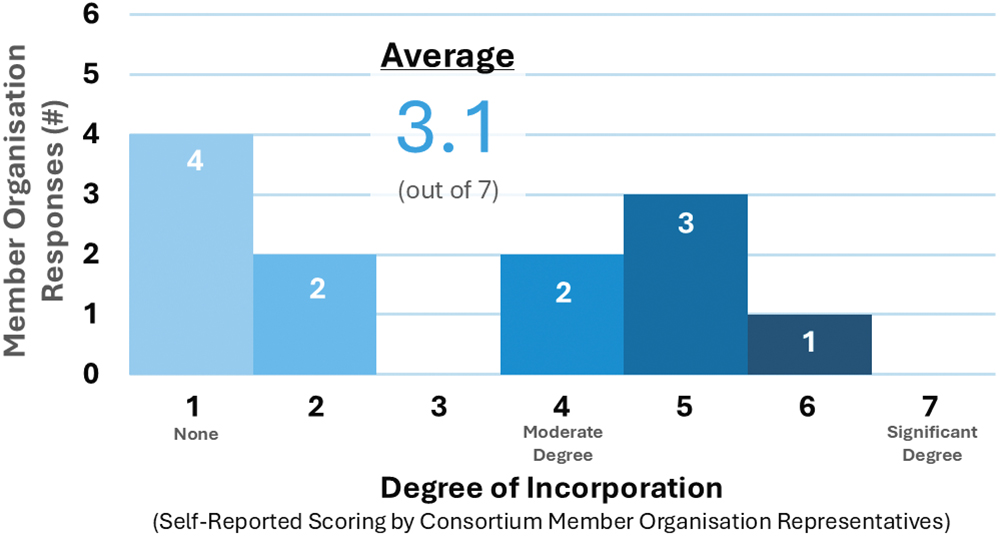
Figure 3: Current incorporation of circularity in Consortium members’ product-development processes.
Safe retrieval of used devices for reprocessing of materials is important to achieving circularity, but study responses indicate that perspectives on take-back schemes are mixed (Figure 4). Consortium member organisations generally acknowledge that successful implementation of reverse-logistics schemes can prevent material losses, but they also point out that they are challenged by high implementation costs and the need to take on greater downstream responsibilities that are beyond the typical reach of pharma companies, including redefining relationships with waste management organisations.
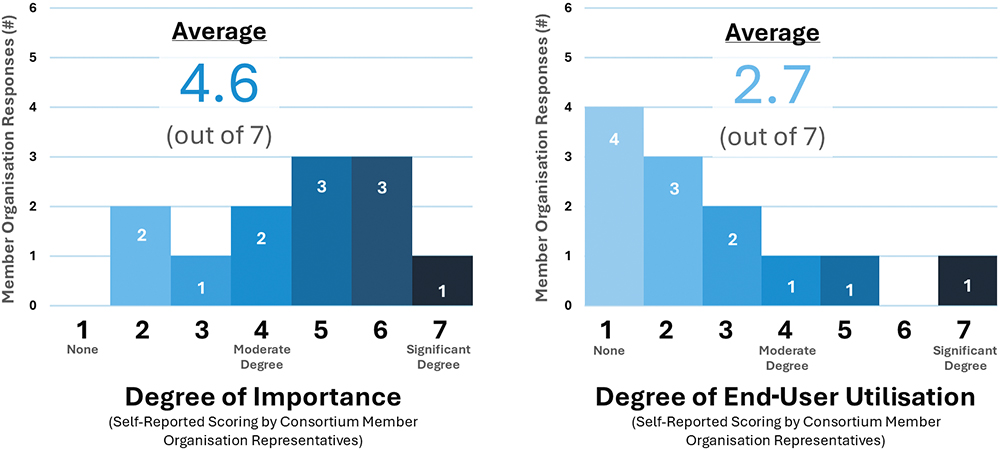
Figure 4: (A) Importance of take-back programmes to achieving Consortium members’ sustainability goals; (B) Current end-user use of Consortium members’ take-back programmes.
Historically, the success rate of medical device take-back schemes has been low,1 and study participants commented that achieving greater return rates requires substantial upfront investment, making it difficult to make a sound business case to support their implementation. However, despite these challenges, some Consortium member organisations are interested in pursuing take-back schemes, especially if there is a stronger and more collaborative push across the industry.
LCAs and Eco-Design Principles
Although lifecycle assessments (LCAs) are conducted for fewer than half of Consortium members’ products, study participants confirm that these assessments are currently used to both assess environmental impact and inform decisions. During development, some Consortium member organisations use internal tools to identify and evaluate opportunities for sustainability improvements. Results from these internal tools are generally not externally reportable but are intended to support decision-making during product design and development phases.
Comprehensive LCA methodologies are typically employed retrospectively for fully defined commercial products, when the overall environmental impact can be calculated and reported. At the time of the study, some Consortium members were not yet conducting LCAs for SC delivery devices. This may present a barrier to these organisations with respect to future collaboration opportunities, including learning best practices from other organisations and aligning on common industry practices for analyses and reporting.
Eco-design – a product-development approach that considers (and aims to reduce) environmental impact throughout a product’s lifecycle – is a noteworthy approach for promoting environmental sustainability. Embedding good eco-design principles in product development can broadly improve sustainability outcomes when designing or updating subcutaneously delivered medicines, including enabling the adoption of less impactful materials; facilitating product recovery, disassembly and recycling; and establishing aligned LCA reporting across the supply chain.
Results from the study revealed that eco-design considerations are seen by Consortium members as important levers for packaging, drug delivery devices and procurement activities, but application of these considerations to formulation design and defining the supply chain is perceived to be more challenging (Table 6). Qualitative responses indicated that applying eco-design principles to drug products and delivery devices is perceived as more complex than it is for packaging, due to regulatory requirements, the need for changes in fill-finish operations, and the inherent difficulty of adapting biologics and other formulations. Participants emphasised that such changes require more time, phasing and investment to implement compared with secondary or tertiary packaging.
| Product Development Process | Importance of Eco-Design Considerations* | Formal Incorporation of Eco-Design Criteria** |
| Product Development Overall | 5.3 | 4.8 |
| Packaging | 5.8 | 5.3 |
| Product Development – Devices | 5.7 | 4.9 |
| Procurement | 5.3 | 4.8 |
| Manufacturing – Devices | 5.1 | 4.2 |
| Supply Chain | 4.8 | 4.0 |
| Product Development – Formulation | 4.7 | 4.6 |
| Manufacturing – Formulation | 4.6 | 4.3 |
Table 6: Importance versus formal incorporation of eco-design criteria in various Consortium members’ product-development processes. *Average score on scale from 1 (not important) to 7 (highly important) **Average score on scale from 1 (not implemented) to 7 (significantly implemented).
Regulatory
Sustainability-led transitions must be managed within the context and constraints of existing and emerging regulatory frameworks. Study participants expressed that Consortium member organisations generally have good understanding of and compliance with regulations for topics such as zero emissions targets and pharmaceuticals in the environment, as these have been established for many years.2,3 On the other hand, greater difficulties can be experienced managing compliance with newer legislation and usage restrictions related to problematic materials, such as PVC and per- and polyfluoroalkyl substances.4,5
The pace of regulatory change is increasing within the pharmaceutical industry and has the potential to impact shared supply chains. Newer regulations, such as the Packaging and Packaging Waste Regulation in Europe, are not always fully compatible with medical and medical device regulations.6 While temporary derogations can provide additional time for achieving compliance, these are typically time-limited and, ultimately, the industry must find ways to meet the growing environmental regulatory requirements. The study results suggest that environmental regulations are seen as either a positive stimulant for industry-wide progress towards environmental sustainability or as significant additional complexity for the industry to manage.
“MANY COMPANIES FACE A GAP BETWEEN AMBITIOUS CORPORATE TARGETS AND THE INTERNAL INVESTMENT REQUIRED TO EMBED SUSTAINABILITY PRACTICES IN SC PRODUCT DEVELOPMENT, ESPECIALLY WHEN BALANCING REGULATORY DEMANDS AND PATIENT NEEDS.”
CONCLUSION
The sustainability benchmarking initiative led by the Sustainability Sub-Team of the SC Consortium found that there is strong commitment to sustainability among the Consortium’s membership. However, while there is a measurable desire to achieve impact – from enhancing packaging sustainability to exploring bio-based materials and circularity – notable challenges remain. Many companies face a gap between ambitious corporate targets and the internal investment required to embed sustainability practices in SC product development, especially when balancing regulatory demands and patient needs. Moving forward, the Consortium will prioritise collaborative efforts towards industry-wide best practices, focus on aligning strategic sustainability ambitions with practical implementation, and encourage targeted investments to overcome the complex challenges of transitioning to less environmentally impactful SC drug products (Figure 5). This collective approach will be essential for achieving meaningful environmental progress while continuing to innovate for improved patient outcomes.
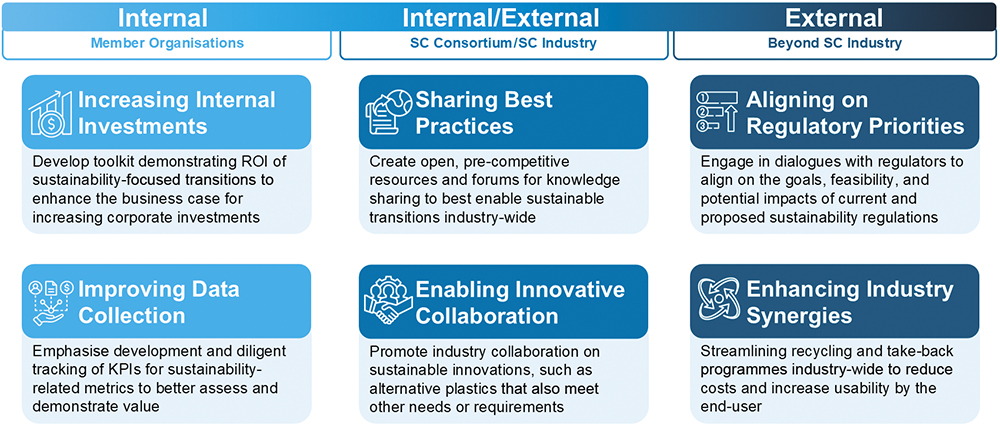
Figure 5: Recommendations following the SC Consortium sustainability benchmarking initiative.
ACKNOWLEDGEMENTS
The authors would like to thank Sascha Rau, QDID – Device Development at Boehringer Ingelheim, and Zahid Nazir, Associate Principal Scientist – New Modalities and Parenteral Development at AstraZeneca, for their contributions to this article.
REFERENCES
- Murphy A et al, “Understanding the feasibility and environmental effectiveness of a pilot postal inhaler recovery and recycling scheme”. NPJ Prim Care Respir Med, 2023, vol 33(1), art 5.
- “Pharmaceuticals in the Environment (PIE)”. Web Page, EFPIA, accessed Sep 2025.
- “Guideline on the environmental risk assessment of medicinal products for human use”. EMA, Aug 2024.
- “Investigation Report on PVC and PVC Additives”. European Chemicals Agency, Nov 2023.
- “EU PFAS restriction”. Web Page, Plastics Europe, accessed Sep 2025.
- “Regulation (EU) 2025/40 of the European Parliament and of the Council of 19 December 2024”. Official Journal of the European Union, Jan 2025.

