To Issue 177
Citation: Buchine B, “Beyond the Barrel: A Dual-Cartridge Improvement to Traditional Dual-Chamber Devices”. ONdrugDelivery, Issue 177 (Sep/Oct 2025), pp 20–25.
Dr Brent Buchine explores how Windgap Medical‘s devices transform dual-chamber systems with a modular, scalable and remarkably simple approach to reconstitution and complex drug delivery.
HAVE DUAL-CHAMBER DEVICES HAD THEIR DAY?
Monoclonal antibodies. Depot suspensions. Long-acting biologics. As R&D pipelines swell with increasingly complex injectable therapies, so does the need for delivery systems that can administer them safely, simply and swiftly at the point of care.
However, while drug formulations have advanced, many of the delivery systems supporting them have not. Once considered a breakthrough, traditional dual-chamber cartridges (DCCs) – glass barrels separated by stoppers with internal bypasses – are now becoming a bottleneck. Their rigid formats, intricate filling and assembly requirements and limited capacity can slow down development, drive up costs and frustrate end-users.
At the same time, dual-chamber pens and autoinjector platforms face their own constraints, often requiring manual shaking, awkward orientations and complicated steps that introduce new risks for patients and providers alike.
“THE INDUSTRY IS AT A TURNING POINT. THE DELIVERY SYSTEMS THAT ONCE REDUCED COMPLEXITY ARE NOW INTRODUCING IT.
FOR BIOPHARMA TEAMS ADVANCING THE NEXT GENERATION OF INJECTABLES, THIS SHIFT DEMANDS MORE THAN A RETROFIT.”
The industry is at a turning point. The delivery systems that once reduced complexity are now introducing it. For biopharma teams advancing the next generation of injectables, this shift demands more than a retrofit. It calls for a fundamental reimagining of delivery architecture, designed to meet modern development demands and patient realities, side by side.
THE CASE FOR EVOLVING THE DUAL-CHAMBER STANDARD
Traditional DCCs were designed to solve a specific problem – shelf life. They do so by storing a lyophilised drug and diluent separately in a single container until the moment of administration. But as therapies grow more complex, this seemingly simple platform introduces friction at nearly every stage of the drug lifecycle.
- Formulations Face Physical Limitations: With drug and diluent housed end to end, teams must work within fixed volumes and ratios dictated by the cartridge’s geometry. This restricts design options and can force compromises in concentration, viscosity, sequencing or dead volume – making difficult trade-offs common in early development.
- Manufacturing Requires a Multistep Fill Process: Where each chamber must be filled, lyophilised, stoppered, filled again and stoppered again, all within a single glass barrel. This sequence demands specialised equipment not widely available in most facilities in addition to uncommon tooling and stringent process controls, all of which extend production timelines and drive up the cost of goods.
- Supply Chain Constraints Introduce Added Vulnerability: Only a small number of vendors supply DCCs at commercial scale and even fewer are equipped to fill them. This scarcity, which shows no signs of abating, can further extend lead times and elevate risk, especially for lean teams managing global supply chains or scaling to commercial volumes.
- The Device Itself: To mix and deliver the drug, DCC-based devices must break internal stoppers and often rely on manual shaking and sometimes strict orientation requirements. When even minor complexity introduces risk, every additional step, component and second can detract from usability and adherence.
While DCCs have played a significant role in extending the application of injectables, the real challenge is the container, with the complexity extending to the formulation, filling and delivery of the drug.
THE PARALLEL DUAL-CARTRIDGE ADVANTAGE
Windgap Medical approaches this challenge from a new angle, literally and architecturally. Instead of filling two stacked substances into a single, complex primary drug container, Windgap uses two standard – and widely available – single-chamber cartridges positioned side by side (Figure 1).
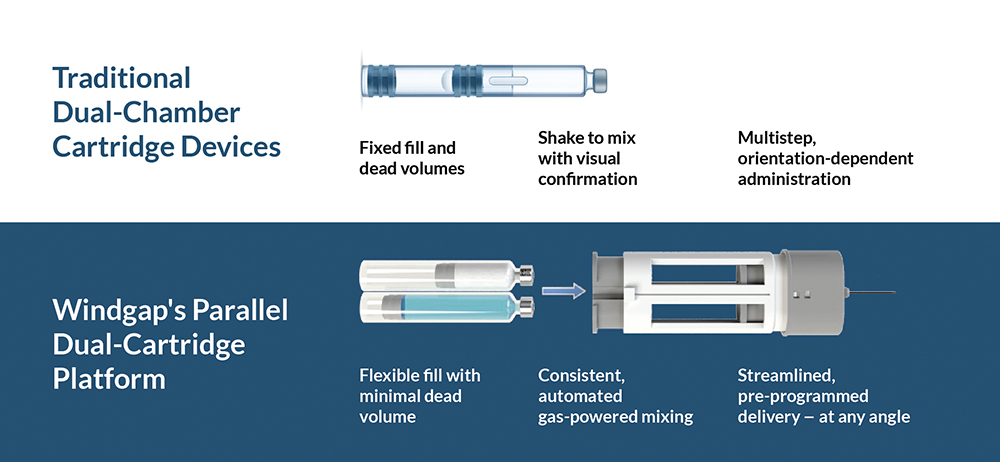
Figure 1: Side-by-side comparison of traditional DCC and Windgap dual-cartridge architectures.
By reimagining both the delivery system and the surrounding ecosystem, Windgap creates opportunities for innovation throughout the product lifecycle.
- Formulation Freedom: Side-by-side geometry removes the constraints of a single, fixed barrel. Teams can mix and match cartridge volumes, contents and timing to support a wide range of drug characteristics from high-viscosity biologics to staged or sequential therapies. This architecture enables flexible dosing, tailored reconstitution and novel delivery strategies that traditional DCCs cannot support.
- Manufacturing Manageability: Each cartridge can be filled independently, with diluents on standard liquid filling lines and lyophilised drugs via protocols similar to vial-filling operations. With fewer integrated steps and no need for custom DCC tooling, the fill-finish process is, by design, easier to validate, less expensive to operate and more adaptable for partners and production scales.
- Supply Chain Flexibility: Windgap’s platform uses ISO-compliant cartridge formats available from multiple vendors, many being offered in ready-to-use configurations in a variety of sizes. This expands sourcing options, shortens lead times and streamlines scale-up across manufacturing partners and sites.
- Smarter Device Mechanics: Instead of relying on internal plungers, springs, or user effort, Windgap’s gas-powered devices allow for reciprocated flow, controlled injection sequencing and instant reconstitution. Full reconstitution protocols can be validated without requiring shaking, swirling or visual guesswork.
The result is a dramatically improved user experience. Completion and proper mixing can be validated. Drugs can be delivered in as few as two steps with no orientation constraints and with clear feedback on successful drug delivery. For patients and providers alike, the result is greater confidence, lower training burden and fewer opportunities for error. Table 1 presents a side-by-side comparison. The technology has pushed the boundaries of what is possible with formulations, feasibility studies and the potential of the platforms.
| Feature | Traditional Dual-Chamber Cartridge | Windgap’s Parallel Dual-Cartridge |
| Cartridge Type | Complex dual-chamber glass cartridge |
Two standard single-chamber cartridges. (1, 1.5, 3 & 5 mL) |
| Filling Complexity | High: fill, lyophilise, stopper-in-place, fill, stopper |
Low: separate liquid fill and lyophilisation |
| Component Supply | Limited vendors, long lead times |
Standard formats from multiple suppliers |
| Device Integration | Single-axis mechanics with complex timing and orientation issues |
Parallel fluid paths, fewer moving parts |
| Reconstitution Flexibility | Low: fixed geometry | High: tuneable for diluent or cake geometry |
| Manufacturing Cost | Higher: complex processes and controls |
Lower: standard lines, decoupled processes |
| Risk During Scale-Up | Higher: long validation cycles | Lower: modular assembly, standard inputs |
| Injection Force Efficiency | Constrained by inline geometry |
Optimised for multi-cartridge force balance |
| Device Size | Long form factor due to single barrel |
Compact layout with improved ergonomics |
Table 1: Comparing technologies – Windgap dual cartridge versus traditional DCCs.
ARCHITECTURE MEETS APPLICATION
Windgap’s parallel cartridge architecture powers a portfolio of delivery platforms, each purpose-built to meet distinct formulation and delivery needs.
DualFlo™: Simplified Sequential Dosing
DualFlo is designed for therapies that require two liquid components to be injected in a specific sequence that cannot be stored together in the same primary packaging. This compact, single-use device delivers the contents of both cartridges through a single needle, in a prescribed order, without requiring multiple devices, needle pricks or added user steps. DualFlo can also support high-volume injections, enabling a full 6 mL dose via two standard 3 mL cartridges in just one injection.
OneMix™: The Instant Solution™
Built for highly soluble lyophilised drugs that dissolve rapidly, OneMix is a true two-step autoinjector – simply remove the cap to mix and press the needle shield to inject. Its simplicity, compact size and single-use format make it ideal for field settings and self-administration of lyophilised therapies. Behind its intuitive simplicity is complex precision. OneMix’s gas-powered mixing and plunger-speed controls are carefully tuned to the therapy and engineered into the device during manufacturing, ensuring consistent delivery of even shear-sensitive biologics, with reduced complexity for the user.
MultiMix™: Complex Mixing, No Shaking
MultiMix is built to handle difficult formulations – including high-viscosity biologics, long-acting injectables and depot suspensions. Its three-step design initiates a pre-set series of gas-powered reciprocation cycles between the two standard cartridges with no shaking required. Once mixing is complete, the device provides a clear signal that it is ready for injection – simply remove the cap and press the trigger against the injection site. By delivering complete, consistent and confident mixing in a fraction of the time, MultiMix eliminates guesswork, prolonged setup and the need for specialised training.
DuraMix™: Reusable, Reliable, Ready for What’s Next
DuraMix brings the power of MultiMix into a reusable, electromechanical platform designed for chronic care and digital health integration. With a durable outer housing and a replaceable dual-cartridge subassembly, it delivers a rare combination of flexible configuration, precise control and consistent performance across doses. DuraMix also supports data logging and connectivity – enabling integration with digital health systems, remote monitoring platforms and personalised care protocols.
Windgap’s technology provided an unprecedented level of control and improvement, enabling the successful administration of a difficult-to-mix 75 cP suspension through a 29G XTW cannula. This reduced the combined mixing and delivery time by 99%. Such a level of efficiency will have significant implications for complex formulations.
“WINDGAP’S DUAL-CARTRIDGE PLATFORM IS NOT JUST A MECHANICAL IMPROVEMENT – IT IS A LIFECYCLE ADVANTAGE, DESIGNED TO REDUCE DEVELOPMENT AND MANUFACTURING FRICTION FROM FORMULATION TO FINAL DOSE.”
THE SMARTER PATH FROM DEVELOPMENT TO DELIVERY
Windgap’s dual-cartridge platform is not just a mechanical improvement – it is a lifecycle advantage, designed to reduce development and manufacturing friction from formulation to final dose (Figure 2).
- Early Development – Flexibility and Forward Progress: Standard cartridge formats allow formulation teams to iterate quickly and run preclinical studies without the need to lock complex packaging. Reconstitution steps can be validated early, accelerating regulatory filings and the path to first-in-human trials.
- Manufacturing – Streamlined and Synchronised: As drug and diluent are filled separately, teams can work across an established, trusted and validated supply base – easing co-ordination with CDMOs and minimising delays.
- Commercialisation – One Format, Many Front Doors: Having a common dual-cartridge platform makes it easier to launch device variants for multiple indications, dosing regimens and patient populations – without starting from scratch. This reduces the time to market, cost of goods, complexity and overall risk.
- Patient and Provider Use – Simplicity that Scales: Most importantly, these devices are designed with the end-user in mind. Both patients and healthcare providers benefit from fewer steps, shorter preparation times and less opportunity for error. This leads to lower risk and improved outcomes, whether they’re at a clinic, a kitchen table, or anywhere in between.
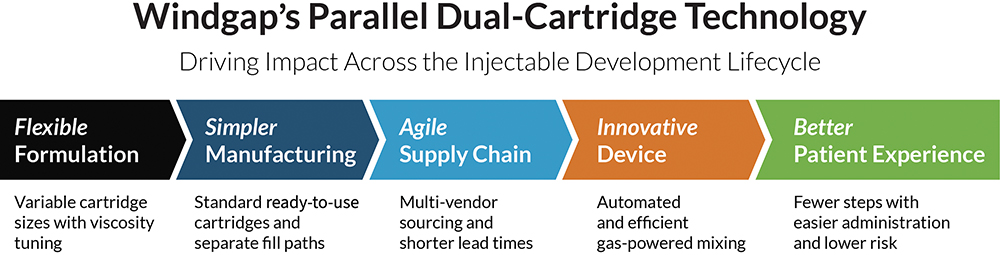
Figure 2: End-to-end advantages of Windgap’s parallel dual-cartridge system.
Few platforms deliver measurable benefits across development, manufacturing and user experience. Windgap’s does, by design.
CONTAINING COMPLEXITY: SOLVING THE NEED FOR MODULAR, SCALABLE AND SIMPLE
Modern injectable therapies are more viscous, more sensitive and harder to deliver than ever before – and the pressure to mix and administer them safely at the point of care is only growing.
Conventional thinking says rising complexity demands more complex devices. In fact, the more challenging the formulation, the more critical it becomes to have a system that simplifies delivery – creating new opportunities for formulation teams, patients and the industry as a whole.
The industry has long sought a platform that does more than deliver drugs. By rethinking the architecture at the core, Windgap’s platforms show how a single innovation can streamline formulation, simplify manufacturing, improve sustainability and elevate the patient experience.
By building on the trusted principles of dual-chamber delivery, Windgap’s platform evolves a familiar foundation into a modern solution, one designed to meet today’s complexity and tomorrow’s breakthroughs with greater ease, flexibility and scalability.

