Citation: “Enabling Subcutaneous Delivery of Biologics”. ONdrugDelivery Magazine, Issue 97 (May 2019), pp 6-9.
Subcutaneous delivery of monoclonal antibodies is typically limited by viscosity-associated syringe forces and poor stability. Elektrofi introduces Elektroject™, a gentle process for the production of ultra-high concentration protein formulations, that maintains a syringeable format and excellent protein stability, making the switch from intravenous to subcutaneous delivery viable for numerous biotherapeutics, including monoclonal antibodies.
Monoclonal antibodies (mAbs) are expected to reach combined global sales of US$150 billion (£116 billion) by 2022 thanks to healthy development pipelines. In recent years, biosimilars and mAbs with similar drug targets have also entered the market, creating a highly competitive landscape and compelling biopharmaceutical companies to differentiate their products.1 Since their initial introduction to the market in 1986, mAb therapies have had tremendous impact, but have yet to reach their full potential, largely because of hurdles in drug delivery.
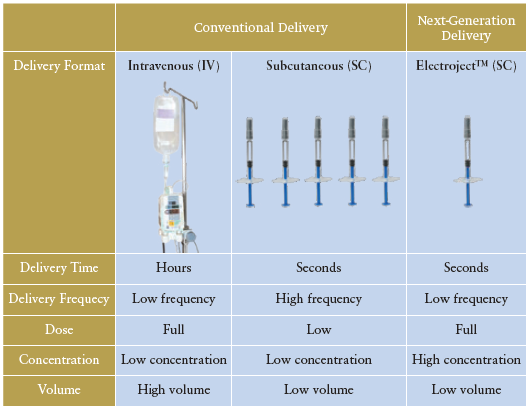
Table 1: Elektroject™ directly addresses challenges in mAb delivery.
Solving delivery challenges will be key to enabling mAb therapeutics to reach their full potential. To achieve optimal therapeutic effect, antibodies often require doses as high as 1 g. These antibodies are conventionally administered by high-volume intravenous (IV) infusions that can last up to eight hours under carefully monitored conditions.2 Such infusions are inconvenient and often financially inaccessible for the patient. Additionally, they can limit the number of patients that hospitals and infusion centers can treat. Subcutaneous (SC) injections of biologics are preferable to IV infusions as they decrease the burden on healthcare providers and payers by requiring much less time and offering a lower risk of complications (infection, infusion reaction, etc.). For patients, they also offer the opportunity for self-administration and favourably alter the economic landscape: SC mAb drugs are much more affordable as they obviate the high mark-ups typical of long-duration IV infusions.3
“Since their initial introduction to the market in 1986, mAb therapies have had tremendous impact, but have yet to reach their full potential largely because of hurdles in drug delivery.”
This preference for SC delivery is reflected in the market as an increasing number of mAb therapeutics have been released in an SC form in recent years.3 For many mAbs, however, the high concentrations (>100 mg/mL) needed to reach appropriate doses within the volume limit of standard SC injections – 1.0 to 2.25 mL – are typically intractable on account of high viscosity-associated syringe force or protein instability.4 High syringe forces make SC delivery virtually impossible, constraining drug manufacturers to either provide IV infusions or administer lower doses more frequently. Elektrofi has overcome these delivery challenges (see Table 1).
ELEKTROJECT™ DIRECTLY ADDRESSES SC MAB DELIVERY CHALLENGES
With large-molecule biologics such as mAbs, protein instability and high viscosities result from intermolecular interactions in solution.4 High viscosities make it difficult to handle and inject the drug. Protein instability reduces the effective dose as less of the protein is therapeutically useful and can potentially form aggregates which may harm the patient.
Strategies for solving these problems include:
- addition of excipients to reduce the prevalence of intermolecular interactions
- administration using on-body injectors to infuse a large volume of low-concentration drug over a prolonged period
- use of hyaluronidase to enable high-volume SC injections.
However, these methods still have not achieved high concentrations without compromising on stability and may require relatively complex administration procedures (i.e. the addition of hyaluronidase still requires a nurse to keep a needle in the patient for 5-6 minutes), which preclude the potential for self-administration.
Elektrofi has developed a next-generation microparticle-based suspension formulation, Elektroject™, which directly addresses the current challenges of SC mAb delivery by enabling ultra-high concentrations (>400 mg/mL) of protein, while maintaining a syringeable format and excellent protein stability.
The Elektroject™ manufacturing process is inherently scalable and can be run aseptically at low temperature. This novel droplet formation and drying process yields dense, spherical microparticles without compromising protein quality (see Figure 1a). The solid microparticles limit the intermolecular interactions responsible for high viscosities and instabilities in aqueous formulations (see Figures 2b and c).
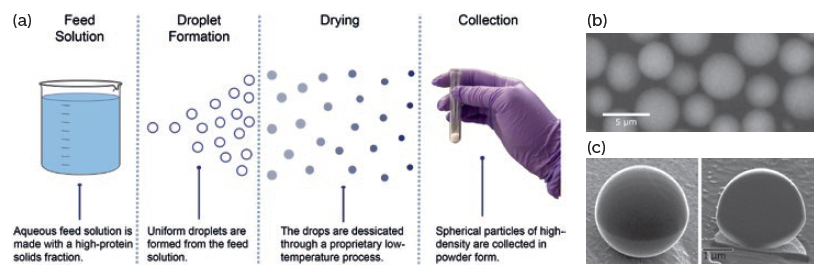
Figure 1: Elektroject™ microparticle production process (a), microparticles (b), and a cross section of a single microparticle demonstrating non-porous morphology (c).
The particles are suspended in a liquid carrier vehicle to prevent dissolution until injection. This suspension can be filled in a prefilled syringe format, eliminating the need for complex, error-prone reconstitution procedures. The highly dispersible nature of the microparticles enables easy resuspension by gentle shaking, allowing for a patient-friendly SC injection. Within the subcutaneous space, the proteins comprising the microparticles readily return to their original monomeric state enabling full bioavailability. Compared with other microparticle formation methods, Elektroject™ formulations:
- do not compromise on protein quality
- achieve higher protein loading
- exhibit tightly controlled particle size and shape.
Altogether, this allows the mAbs to be delivered in a prefilled syringe format and allows SC injection to become the standard of drug delivery for mAbs.
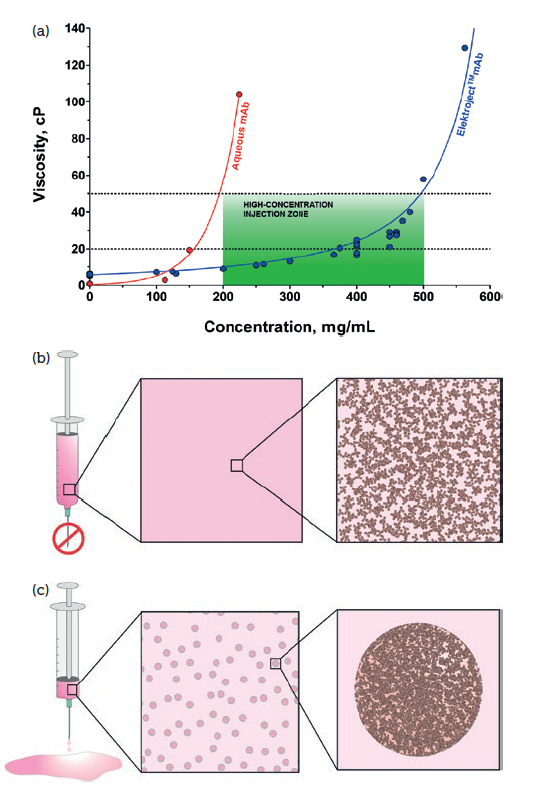
Figure 2: Elektroject™ enables high concentrations of biologics at low viscosity. Graph’s x-axis shows protein concentration for aqueous mAb and particle concentration for Elektroject™ mAb (a). Intermolecular forces drive viscosity and protein degradation at high viscosity (b). Elektroject™ formulations physically reorganise protein solutions into reversible suspensions to reduce viscosity (c).
HIGH-CONCENTRATION, SYRINGEABLE SUSPENSIONS
Viscosity plays an important role in the handling and administration of injectable products. For suspension products, higher viscosities can prevent settling of the suspension. But when the viscosity is too high, it may be too difficult to deliver the drug through a 27-gauge needle because it takes much more force to actuate the syringe. The alternatives, such as using a wider needle or requiring longer injection times, reduce patient compliance with their treatment. Although there is no exact limit on viscosity, since it depends on the patient population and the syringe components, common targets are 20 and 50 cP.
Microparticle size and dispersity also impact syringeability. Microparticles are often recommended to be at least 3-10 times smaller than the inner diameter of the needle. Even if a small fraction of the particle population is larger than that, they may clog the needle and cause the whole dosage to go to waste.5,6
Compared with aqueous mAbs, Elektroject™ suspensions achieve much lower viscosities at high concentrations (Figure 2a). The tight particle size distribution control afforded by the Elektroject™ microparticle production process allows for the use of smaller needles without the risk of clogging (Figure 1b).
Compared with other particle production techniques, such as spray drying, atmospheric spray freeze drying, or polymer-based microspheres, Elektroject™ can make solid, dense microparticles with high protein loading under gentle conditions. While other particle production techniques use high temperatures, which can damage the protein, the Elektroject™ platform operates at low temperatures. Protein loading plays a role in the effective concentration-viscosity relationship. The lower the protein loading, the lower the effective protein concentration, which shifts the suspension curve to the left in Figure 2a. The more excipient is loaded into the solid fraction, the higher the viscosity at any given protein dose.
Elektroject™ can achieve protein loadings greater than 90% while maintaining stability of the protein, whereas other microparticle technologies require relatively large fractions of stabilisers or protectants.
Elektroject™ microparticles are produced to fit through 27-30-gauge needles without needle clogging events. When in a syringe, the Elektroject™ suspensions take up to two hours to sediment and can be easily resuspended with gentle shaking. With these suspensions, protein concentrations of protein concentrations in excess of 400 mg/mL below 50 cP are possible, enabling the possibility of SC delivery.
All this can be done without compromising molecule stability.
MAINTAINING MOLECULE STRUCTURE
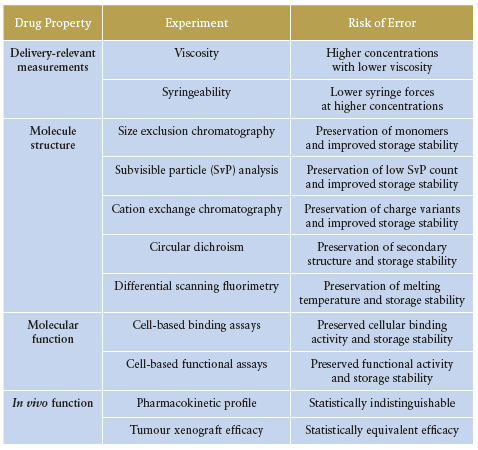
Table 2: Summary of Elektroject™ capabilities.
Elektroject’s gentle particle formation conditions allow for a variety of molecules to be formed into microparticles, including fragile molecules such as mAbs and fusion proteins. Unlike other techniques, Elektroject™ does not require high temperature conditions. Elektroject™ has demonstrated high preservation of mAb structure and functional bioactivity throughout the manufacturing process.
Once the suspension mixes with aqueous media, complete dissolution occurs within seconds to minutes, mitigating any immunological risks posed by particles persisting in the subcutaneous space.
Compared with an equal dose of aqueous mAb, Elektroject™ mAb demonstrated similar pharmacokinetic profile (AUC, Cmax, and Tmax) and efficacy (tumour growth reduction) in an animal model. Table 2 contains a list of highlighted drug properties comparing Elektroject™ particles with aqueous drug.
CONCLUSION
Patient-friendly products will continue to define the future of biologics. Although mAb therapeutics already comprise a large part of the biopharmaceutical landscape by market share, patient accessibility remains a problem. Elektrofi’s next-generation delivery platform, Elektroject™, improves accessibility by enabling the SC delivery of most protein therapeutics at full dose and without compromise on protein quality.
REFERENCES
- Grilo AL, Mantalaris A, “The increasingly human and profitable monoclonal antibody market”. Trends in Biotechnology, 2019, Vol 37(1), pp 9-16.
- Janssen Biotech, “Darzalex (daratumumab) [FDA label]”. 2019.
- Viola M, et al, “Subcutaneous delivery of monoclonal antibodies: How do we get there?”. J Controlled Release, 2018, Vol 286, pp 301-314.
- Bussemer T, et al, “Approaches in subcutaneous delivery of monoclonal antibodies”. Eur Pharm Rev, 2016, Vol 4, pp 26-31.
- Miller M, Engstrom J, Ludher BS, Johnston KP, “Low Viscosity Highly Concentrated Injectable Nonaqueous Suspensions of Lysozyme Microparticles”. Langmuir, 2010, 26(2), pp 1067-1074.
- Puthli S, Vavia P, “Stability Studies of Microparticulate System with Piroxicam as Model Drug”. AAPS PharmSciTech, 2009, Vol 10(3), pp 872.

