To Issue 140
Citation: Rogueda P, “Delivering Biologics with Soft Mist Inhalers – a Market Poised to Expand”. ONdrugDelivery, Issue 140 (Nov 2022), pp 45–48.
Philippe Rogueda discusses the inhalable biologics market, including Merxin‘s successfully approved and marketed products, how the introduction of soft mist inhalers represents a significant opportunity for new and old therapeutics alike, and how the market is poised for a major expansion in therapeutic application, size and value.
“It is important to remember that delivery of biologics via inhalation is not a new idea; in fact, it has been around for decades.”
A frequently discussed segment of the pharmaceutical market is the rapidly growing area of biopharmaceutical therapeutics. This covers a broad range of molecules and a wide variety of disease areas. Biologics are typically more fragile than traditional small molecules, being highly unstable under adverse conditions, and the optimal way to deliver them remains an open question.
Biologics have primarily been the purview of parenteral delivery due to the need to avoid the harsh conditions of the gastrointestinal tract, which many biologics are unable to weather. Another factor is that the synthesis of biologics typically yields aqueous “brews”, which makes liquid formulations much more attractive. However, the issues with regular injections of biologics are well documented, with formulations struggling to keep to acceptable volumes and viscosities. Therefore, there is significant interest in finding alternative ways to deliver biotherapeutics, particularly via the inhalation route.
A PROVEN MARKET
It is easy to see why delivering biologics to the lungs is an attractive option for drug developers – inhalation offers numerous advantages compared with oral and parenteral delivery routes. The inhalation route is non-invasive and needle-free, making it naturally more patient-friendly; is suitable for both local and systemic delivery, giving it potential for a wide array of therapeutics; and most inhalers are highly portable and suitable for at-home use, supporting current drives to move care out of the clinic and into patients’ homes. Additionally, the inhaled route also has pharmacokinetic responses aligned with the parenteral route, further easing a shift from one to the other.
Taking these various advantages into consideration, it is not surprising that a popular pharma market database reports that there are over 700 companies that have had at least one inhalable biologic project in their pipeline, ranging from small biopharma start-ups to major pharmaceutical companies. Similarly, one has only to look to find a wealth of discussions, reviews and research articles concerning inhalable biologics in current scientific literature. The potential is clearly there for a major expansion in this market.
“SMIs offer the advantages of both DPIs and nebulisers, without the drawback of having to formulate the drug as a dry powder, or the inconvenience and lack of protection of nebulisers.”
However, it is important to remember that delivery of biologics via inhalation is not a new idea; in fact, it has been around for decades. To date, there have been four inhalable biologic products approved and marketed:
- Survanta (beractant) by AbbVie – launched 1991
- Pulmozyme (dornase alfa) by Roche – launched 1993
- Afrezza (insulin) by MannKind Corporation – launched 2014
- Exubera (insulin) by Pfizer – launched 2017.
These approvals demonstrate that regulatory pathways exist for inhaled biologics to reach the market. With three of these products still going strong, coupled with recent advances in inhalation technology and the recent flourish of new biomolecules, this proven market is poised to expand – all it will take is the right product in the right device.
THE RIGHT PRODUCT IN THE RIGHT DEVICE
Of the currently marketed inhaled biologics, two use an inhalation delivery device: Afrezza is delivered via a single-dose capsule-like dry powder inhaler (DPI) and Pulmozyme via a nebuliser. However, a survey of inhalable biologic development programmes from 2020/2021 literature shows a clear bias towards nebulisers as the delivery device of choice (Figure 1).1–3
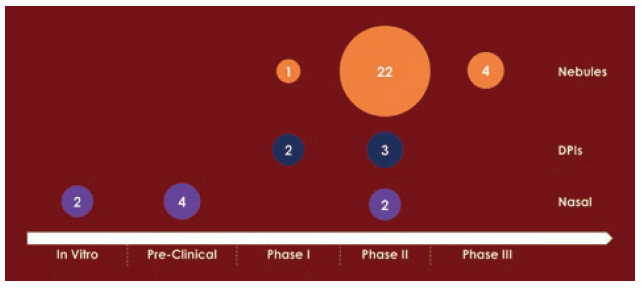
Figure 1: A survey of 40 inhaled biologic development programmes from 2020/2021 literature.
While nebulisers have their advantages, especially in a clinical setting and for emergency care, it is difficult to justify them as the optimal choice for the current market, due to their limited intellectual property (IP) protection, high interchangeability, long dosing times and a high level of drug wastage, which is of particular concern for expensive biologic therapies.
“With the breadth of interest in inhalable biologics, there is little doubt that an SMI such as MRX004 will be a boon to the drug development process, covering all development stages with a single device.”
This poses an obvious question: if other delivery devices are better suited to chronic at-home use, why are nebulisers so popular in early-phase clinical trials? The answer is simple – convenience and availability. The majority of biologics are synthesised as an aqueous or ethanol solution, and so are easily formulated for nebulisation. Additionally, some of the disadvantages of nebulisers for a marketed product are obviated – or turned into strengths – at the clinical trial phase. This makes nebulisers the default choice for drug developers whose priority is to get their therapeutic into trials as soon for as possible for a quick clinical signal.
The allure of nebulisers for clinical trials becomes even clearer when the difficulty of formulating a biologic for a DPI is considered. Preparing a dry power formulation for a biologic is far from trivial. The challenges include the need to process the biologic molecules into a powder form without damaging them, keep them in humidity-controlled storage and avoid electrostatic charging within the primary packaging and the device, among other key considerations.
However, despite this perceived convenience, going into clinical trials with a nebuliser is often a strategic error. As a project progresses through the clinical trials process with a nebuliser, it is going to encounter a dilemma – do you go the whole way with the nebuliser and go to market with a suboptimal drug-device pairing? Or do you pick a point to throw out the progress made with the nebuliser and start from scratch with a DPI?
As such, it is not surprising that other potential delivery devices are becoming the preferred choice. The ideal device should have strong market protection, be suitable for delivering sensitive biologics in aqueous or ethanol-based solutions and have proven patient usability and acceptability. There has been some interest in the nasal route, but it is not a substitute for delivery to the lungs. Nasal delivery’s strengths lie in one-off acute and emergency treatments, where pure simplicity in delivery is a major concern, rather than delivering medication for chronic indications, where patients have more time to learn and become accustomed to the device.
But if not DPIs, nebulisers or nasal delivery, what? Recent years have provided the answer to this question with the development of a new inhaler device category that fits the bill perfectly – the soft mist inhaler (SMI).
SOFT MIST INHALERS – UNLOCKING THE POTENTIAL OF INHALABLE BIOLOGICS
When developing a biologic for inhalation, SMIs offer the advantages of both DPIs and nebulisers, without the drawback of having to formulate the drug as a dry powder, or the inconvenience and lack of protection of nebulisers. With SMIs, the term “soft mist” refers to both the aerosol-generation mechanism and the quality of the aerosol itself; SMIs are functionally non-pressurised metered dose inhalers that use a mechanical power source, such as a compressed spring, to push a liquid formulation through a microfluidic nozzle to create a fine, slow-moving aerosol suitable for inhalation, allowing for easy delivery in distinct, metered doses.4 Due to their use of liquid formulations, SMIs are readily suitable for aqueous and ethanol-soluble biologics in the same way that nebulisers are. Having significant advantages at both the clinical and market stages, SMIs are poised to be a game changer for inhalable biologics.
Similar to DPIs, SMIs are portable, pre-dosed, do not require patients to handle the formulation, offer strong IP protection and do not require an external power source. From the nebuliser side, SMIs use a solution formulation and are relatively quick to get to clinical trials. SMIs are accurate and capable of reliable deep-lung drug deposition; by changing the size of the pores in the mesh, it is possible to control the size of the droplets delivered to the patient and therefore the region of the lungs in which most will be deposited.
Another advantage of SMIs is the high fine particle fraction (FPF) they can achieve. This means that a significantly greater proportion of the drug can reach the target region of the lungs compared with other inhalation devices. Therefore, less drug is required to achieve the same therapeutic effect and less overfill of the device is required, which is a significant benefit when dealing with expensive biologic molecules.
The first SMI to reach the market was Boehringer Ingelheim’s Respimat®, which is now used to treat a variety of respiratory indications, including asthma and chronic obstructive pulmonary disease (COPD). The success of Respimat has demonstrated that there is an appetite for SMIs across the market, with approval from regulators and a positive response from patients and healthcare providers.

Figure 2: Merxin’s MRX004 soft mist inhaler (www.mrx004.com).
With Respimat’s patent expiring, there is now a prime opportunity for a massive expansion in SMI use as competitor devices enter the market, giving drug developers more device options to choose from to differentiate their drug and optimise its delivery. With inhalation device specialists able to offer generic and customised SMIs, the potential of this device category is ready to be unlocked.
Leading the way in the expansion of SMIs is Merxin, which has developed the MRX004 SMI (Figure 2). Initially, MRX004 has been developed and optimised for tiotropium olodaterol formulations for the maintenance treatment of COPD. However, the potential of MRX004 is far greater than COPD alone; it is ideal for both new molecules and lifecycle management of existing inhalable products. For example, Merxin has formulated Pulmozyme for the MRX004 and achieved a 60% FPF (<5 μm), demonstrating that it is an ideal candidate for reformulation for an SMI (Figure 3), as well as nicotine for nicotine replacement therapies and cannabidiol.
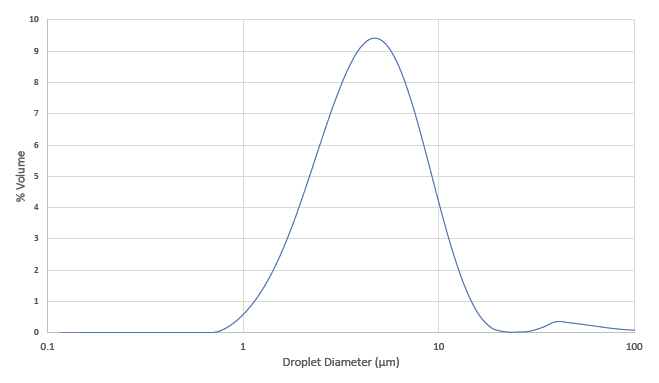
Figure 3: Average droplet size distribution of dornase alfa formulated for MRX004.
With the breadth of interest in inhalable biologics, there is little doubt that an SMI such as MRX004 will be a boon to the drug development process, covering all development stages with a single device – from in vitro screening to clinical trials and commercialisation. An SMI can be used from the first clinical signal to the final product, meaning that there is no concern about duplication of effort or the need to choose the right time to switch devices.
Partnering with a device expert enables projects to unlock the potential of their formulations by ensuring that they are paired with the optimum device. Merxin helps guide its partners through the device selection process, such as determining if the best option is a generic device or one specifically customised to the drug, in order to meet their development goals.
POISED TO EXPAND
With new device players ready to increase the presence of SMIs, the market for inhaled biologics is poised to expand significantly in both size and value. Inhaled biologics have enormous potential for the treatment of respiratory diseases, such as asthma, COPD and covid-19, as well as exciting prospects for a variety of systemically acting therapeutics, such as insulin, antibiotics, anti-infectives and vaccines.
Because of this diversity, it is challenging to make any firm predictions of how the market will grow and where the largest emphasis will be. It can be expected that, once its expansion gets underway, the inhalable biologics market will borrow growth from other sectors, and as the advantages become more self-evident, an increasing number of products will gain regulatory approval as patients come to favour the ease and convenience of inhaled therapies over their parenteral competition.
For both biopharma companies developing new molecules and established brands looking for lifecycle management options for their existing products, SMIs represent a major opportunity. As patient friendly devices that are easy to formulate for and offer strong patent protection, SMIs stand out among the inhalation device competition as the go-to device for inhaled biologics.
To make the most of the opportunity that SMIs and the upcoming expansion of the inhaled biologics market offers, it is important to partner with a device specialist to ensure that the optimum drug device pairing is made. With a proven track record in both generic and custom devices, including single- and multidose DPIs and its MRX004 SMI, Merxin is ideally placed to provide the technology and expertise that an inhaled biologic development project needs to succeed.
REFERENCES
- Liang W et al, “Pulmonary Delivery of Biological Drugs”. Pharmaceutics, 2020, Vol 12(11), Article 1025.
- Matthews AA, Ee PLR, Ge R, “Developing Inhaled Protein Therapeutics for Lung Diseases”. Mol Biomed, 2020, Vol 1, Article 11.
- Parray HA et al, “Inhalation Monoclonal Antibody Therapy: A New Way to Treat and Manage Respiratory Infections”. Appl Microbiol Biotechnol, 2021, Vol 105, pp 6315–6332.
- Leiner S et al, “Soft Mist Inhalers” in “Pharmaceutical Inhalation Aerosol Technology, Third Edition” (Hickey AJ, dr Rocha SRP, eds). CRC Press, 2019.

