Citation: Morchain B, Cordier S, Verger E, “Ensuring Functional Performance and Regulatory Compliance of Elastomer Stoppers for Multipiercing Situations, ONdrugDelivery, Issue 120 (May 2021), pp 68–71.
Bruno Morchain, Sébastien Cordier and Estelle Verger discuss a series of experiments conducted on Aptar Pharma’s PremiumCoat® vial stoppers to demonstrate their compliance with EU and US Pharmacopeia standards and their excellent performance in multipiercing situations.
When developing a drug product, be it a new drug, a generic or repurposing an existing drug, specifying the appropriate primary packaging is an essential step. Because it is in direct contact with the drug product, the primary container plays a critical role in preserving the drug’s integrity throughout its lifecycle, from the initial point of production, through packaging, distribution, short- or long-term storage, up to the moment when it is administered to the patient. As such, any failure associated with the primary container has the potential to result in serious adverse effects for the patient or the healthcare practitioner, which, aside from the potential significant human impact, can also result in financial implications for the drug manufacturer.
“Sharpness may become problematic when the needle punctures the elastomeric vial stopper as it may tear off fragments, which will end up in the drug solution and may compromise patient safety.”
Primary drug packaging for injectables is typically comprised of two parts: a glass container for holding the solution and an elastomeric component. In applications using vials, further to requirements that the container can be closed easily during the filling process and that container closure integrity be maintained throughout the product’s lifecycle, the elastomeric stopper plays a critical role in facilitating easy and safe collection of the drug product by healthcare practitioners.
In the majority of cases, elastomeric stoppers are not removed from the vial in order to preserve the integrity of the drug product. Instead, a needle is inserted through the stopper to access the drug. However, this piercing action can lead to critical incidents, making the stopper’s functional performance key in protecting both the drug and the patient.
In the first instance, the needle must pierce through the elastomer. Although the design of the needle contributes to reducing the insertion force required, the stopper material also plays an important role in easing the act of insertion. Furthermore, the use of hard rubber may bend the tip of the needle and increase patient discomfort during the injection.
An additional risk to patient safety is through coring (tearing off small fragments of the elastomer when the needle pierces the rubber), which leads to contamination of the drug solution. Furthermore, since glass vials can be used to package multiple doses, often the stopper must be pierced several times while maintaining a seal after the delivery of each dose. Elastomeric solutions providers, therefore, need to ensure that the self-sealing capabilities of their components continue to preserve the drug’s integrity over time, avoiding the potential for contamination and oxidation that would be detrimental to patient safety. This is particularly true in the current covid-19 context, where the need to mass-vaccinate large populations has led pharma companies to choose multidose vials as their favoured type of vaccine container.
Over the course of 50 years of collaboration with pharma and biotech partners, Aptar Pharma has developed expertise to address such drug delivery challenges, working with vaccines, biotech drugs and small molecules. Aptar Pharma’s latest innovation is PremiumCoat®, which combines start-of-the-art elastomer formulation with market-proven ethylene tetrafluoroethylene (ETFE) film-coating technology. Under testing, the functional performance of PremiumCoat® stoppers was shown to be fully compliant with the quality controls specified by EU Pharmacopoeia (EP) 3.2.9, demonstrating excellent properties for protecting drugs and patients, and facilitating the drug development process.
“The need to mass-vaccinate large populations has led pharma companies to choose multidose vials as their favoured type of vaccine container.”
PREMIUMCOAT®: REDUCING PIERCING FORCES
When working with vial containers, healthcare practitioners need to pierce the elastomeric closure components to access the drug. The force required to insert the needle depends on factors such as needle diameter and tip design, but also on the mechanical properties of the elastomer. While this force is usually not a limiting factor for the health practitioner’s activity, it may affect the integrity of the needle tip. Over the decades, injectables companies have worked to develop needle designs that minimise patient discomfort during an injection. This is usually achieved by using thinner needles or by increasing the sharpness of the tip. However, sharper tips and thinner needles are inevitably more fragile and therefore more likely to bend if excessive force is applied when piercing the elastomer. The discomfort and pain associated with a bent needle can be avoided by improving the mechanical properties of the vial’s stopper to help reduce the needle insertion force required.
Aptar Pharma’s experts have evaluated the insertion force required to pierce PremiumCoat® stoppers using a 21-gauge needle, comparing stoppers that were sterilised using steam or gamma radiation processes after two years of ageing (Figure 1). The insertion force was measured using a dynamometer at an insertion speed of 200 mm/min, with recordings taken of the maximum force registered during the insertion. EP 3.2.9 sets the maximum acceptable force at 10 N.
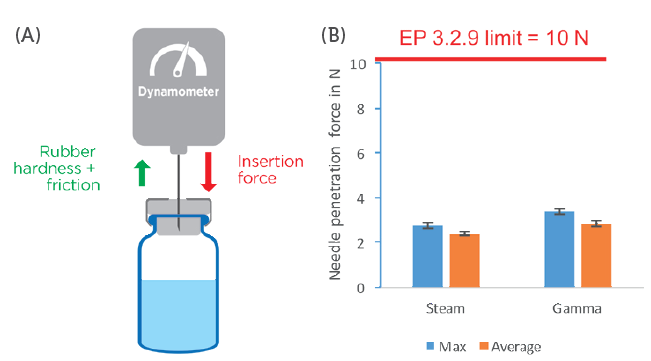
Figure 1: Measurement of needle insertion force. A) Representation of the experimental setup. B) Representation of the maximum insertion force and average insertion force measured for steam- and gamma-sterilised PremiumCoat® 20 mm stoppers. The data were collected after two years of ageing and using 21G 3-bevel needles.
Results showed that, regardless of the sterilisation method used with PremiumCoat® stoppers, the needle insertion force is significantly below the limit imposed by EP 3.2.9. The average insertion force reached 2.4 N (maximum at 2.8 N) for steam-sterilised stoppers, and 2.8 N (maximum at 3.4 N) for gamma-sterilised stoppers.
These results confirm that, despite the presence of an ETFE film, the force required to pierce PremiumCoat® stoppers remains well within EP 3.2.9 and US Pharmacopeia (USP) <381> recommendations. It was shown that PremiumCoat® stoppers are easy to pierce, facilitating ease of the injection process and potentially helping to reduce perceived pain among patients during the injection by preserving the needle tip’s integrity.
PREMIUMCOAT®: PROTECTING THE PATIENT AGAINST FRAGMENT CONTAMINATION
Sharpness is an essential quality for needles, allowing them not only to penetrate effectively through the rubber stopper but, most importantly, to limit the patient’s discomfort during an injection. However, this sharpness may become problematic when the needle punctures the elastomeric vial stopper as it may tear off fragments, which will end up in the drug solution and may compromise patient safety. Therefore, manufacturers must ensure elastomer formulations are less prone to fragmentation to help limit the risk of coring without the need to compromise on the increased patient comfort provided by sharper needles.
Aptar Pharma’s technical team evaluated the performance of PremiumCoat® stoppers in terms of the risk of coring (Figure 2), referring to the limits set out in EP 3.2.9. A total of 12 stoppers were manually pierced four times, each time at a different site. The EP 3.2.9 acceptance limit is set at five fragments over the 12 stoppers and 48 piercing events.
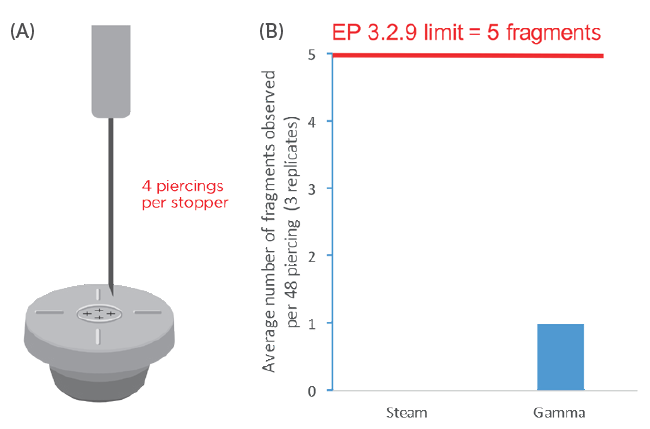
Figure 2: Evaluation of PremiumCoat® 20 mm stoppers piercing fragmentation. A) Representation of the experimental design: each stopper is pierced four times successively at a different spot and the vial’s content filtrated to look for visible fragments. B) Representation of the fragmentation results: the data are represented as the number of fragments per 48 piercing events. Three sets of 12 steam-sterilised and three sets of 12 gamma-sterilised stoppers were each pierced four times with 21G needles.
This experiment revealed that, regardless of the sterilisation method, the number of fragments recorded over 48 piercing events for the PremiumCoat® stopper was very clearly within the acceptance level of EP 3.2.9 and USP <381>. For the steam-sterilised stopper, no fragments were counted after performing the experiment three times (a total of 36 stoppers tested with 144 piercing events). In the case of gamma-sterilised stoppers, either zero, one or two fragments were counted after 48 piercings. After conducting the experiment three times, an average of one fragment was observed per 48 piercings.
The results demonstrate that the risk of rubber fragmentation is very low with PremiumCoat® stoppers and fully in accordance with EP 3.2.9 and USP <381> guidelines. PremiumCoat® stoppers reduce the risk associated with elastomer fragment contamination of drug products, contributing to improved patient safety and safe drug injections.
PREMIUMCOAT®: MAINTAINING OPTIMAL SEALING AFTER MULTIPIERCING
Multidose primary drug packaging is commonly used in the injectables market, especially in the case of vaccination campaigns or in settings that focus on waste limitation and cost savings. In principle, the stopper of a multidose vial will be pierced several times, and each piercing increases the risk of compromising the sealing properties of the stopper. Due to their elastic properties, elastomers demonstrate a level of resilience that allows them to reseal after being breached, and rubber-component providers have been working on specific formulations that ensure long-lasting protection for drugs in multipiercing applications.
BOX 1: APTAR PHARMA’S PREMIUMCOAT®
The function of ETFE films is to form a barrier to protect the drug from extractable and leachable chemicals. However, the presence of a film must not negatively affect the other key properties of stoppers. The results of the tests performed by Aptar Pharma’s expert teams show that PremiumCoat® stoppers are fully compliant with the EP 3.2.9 recommendations. Regardless of the sterilisation method chosen and even in straining circumstances, such as the mass vaccinations for covid-19, PremiumCoat® is a reliable solution that ensures the success of Aptar’s pharma partners by:
• Facilitating easier injections and potentially reducing perceived pain among patients by protecting the needle tip.
• Helping to protect the drug and patients against particulate contaminations, even in a multipiercing context.
• Safeguarding the drug’s integrity throughout its lifecycle, even for multidose applications.
Aptar Pharma’s experts evaluated the ability of PremiumCoat® stoppers to preserve the seal closure in conditions that simulate multipiercing applications (Figure 3). The stoppers were pierced 10 times with the same needle before the vial was submerged into a coloured solution. The system was placed in a vacuum and the pressure reduced. Upon restoring atmospheric pressure, any failures in sealing properties would be highlighted by a colouration of the contents within the vial.
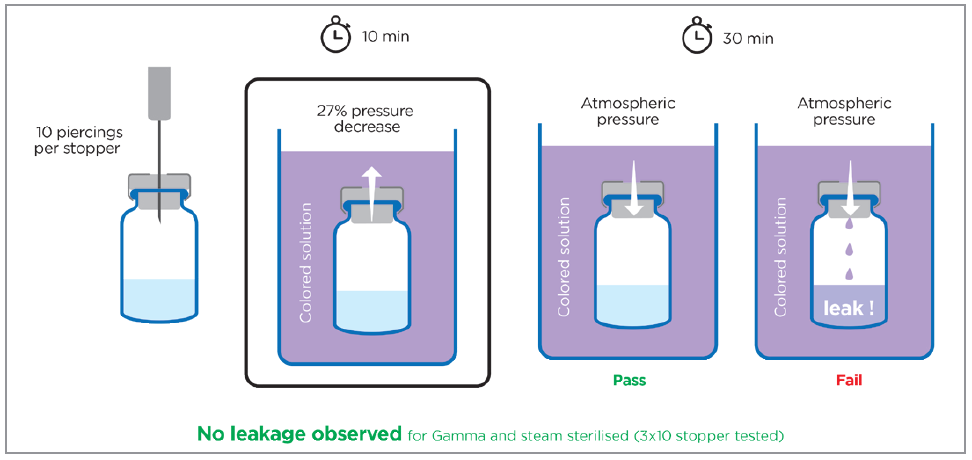
Figure 3: Evaluation of the self-sealing ability of PremiumCoat® 20 mm stoppers. Each stopper was pierced 10 times at a different site with a 21G needle then immersed in a coloured solution while reducing ambient pressure by 27 kPa in a vacuum chamber. Atmospheric pressure was then restored and the inside of the vials were monitored for evidence of potential leaks of the coloured solution. The arrow represents the pressure gradient. A total of 30 observations were performed for both steam-sterilised and gamma-sterilised stoppers.
The experiment clearly demonstrated the self-sealing performance of PremiumCoat® stoppers, with the seal closure maintained even after 10 successive piercings. No leakage was observed among the 60 stoppers tested, and the sterilisation method did not affect the performance of the stoppers.
These results highlight how, even in an extreme case where the rubber is pierced 10 times, the self-sealing capabilities of PremiumCoat® stoppers maintain container closure integrity. PremiumCoat® stoppers, therefore, provide an optimal solution for preserving the drug product – even in multidose applications – and ensuring patients receive safe injections (Box 1).
All data referenced in this article are from Aptar Pharma’s internal report #TR20-0206, conducted in 2020 in Villepinte, France.
For more information about PremiumCoat®, visit: www.aptar.com/products/pharmaceutical/premiumcoat-coated-vial-stoppers-and-syringe-plungers.


