To Issue 158
Citation: Dantas C, Krueger U, “Exploring the Potential of Nebulised Biologics”. ONdrugDelivery, Issue 158 (Apr 2024), pp 12–14.
Carolina Dantas and Ulf Krueger consider the challenges when it comes to the successful delivery of inhaled biological drugs.
Biologics, administered via inhalation, offer the possibility of direct administration to the targeted site of activity. This route offers advantages such as direct drug delivery to specific lung regions, increased local concentrations, reduced systemic side effects and less-invasive treatments leading to improved patient adherence. As the pharmaceutical industry explores alternative routes for drug administration, the potential of nebulised biologics in treating respiratory and systemic diseases continues to garner attention, promising effective personalised treatments.
BIOLOGICS – AN OVERVIEW
Generally, biologics are therapeutic proteins derived from living systems (such as bacteria, yeast, plants or animals) or blood that differ from “small molecules”, which are synthetic, or plant derived (see Figure 1). Examples of biologics include vaccines, growth factors, monoclonal antibodies and immune modulators. With their high target specificity and comparatively low toxicity, biologics are one of the fastest-growing therapeutic classes in the pharmaceutical industry.
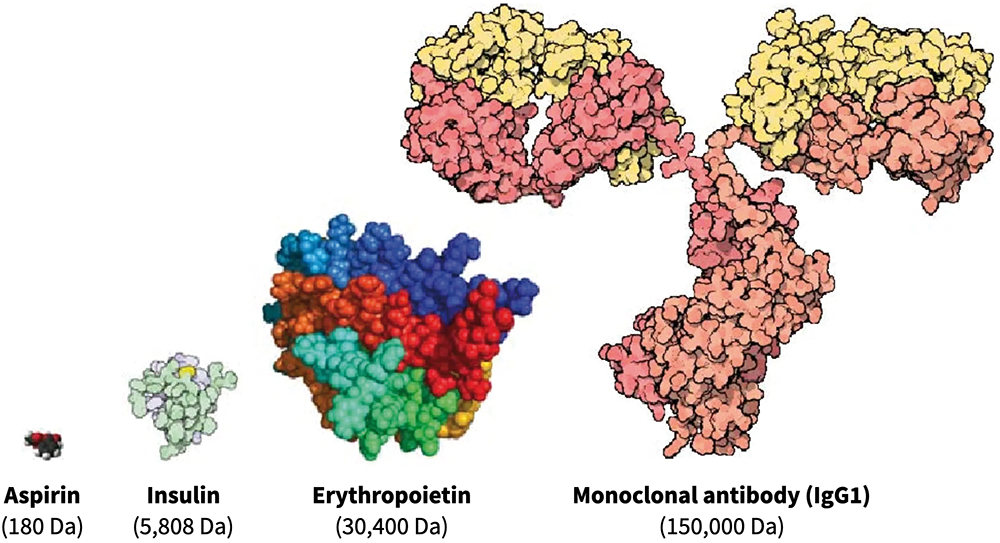
Figure 1: Comparison of three different classes of biologic drugs with a typical small molecule. (Adaptation from Revers L & Furczon E, Canadian Pharmacists Journal, 2010.)
While traditionally administered via intravenous injection, advancements have sparked interest in exploring alternative routes, particularly inhalation, for the treatment of not only respiratory diseases but also systemic conditions.
In the context of respiratory diseases, biologics exert their therapeutic effects through different mechanisms of action, including modulation of immune responses, inhibition of inflammatory pathways and targeting specific cellular receptors involved in disease pathogenesis.
The growing interest in the inhaled route for biologics is related to:
• Direct drug delivery in the targeted lung region (e.g. locally in the lungs or systemic delivery)
• Increasing local drug concentrations with reduced doses
• Reduction of toxicities and side effects
• Non-invasive administration, leading to better patient adherence and convenience
• Reduction of costs and risks in clinical development.
“The formulation of biologics for inhalation poses significant challenges due to their larger size, complex structure and unique properties.”
Despite these advantages, the formulation of biologics for inhalation poses significant challenges due to their larger size, complex structure and unique properties. Addressing these challenges requires thorough strategies because formulation modifications that target specific uptake routes increase mucus permeation, prevent degradation or improve resistance to aerosolisation. Froelich and Salar-Behzadi (2021) have highlighted several approaches to optimise formulations for the inhalation of biologics, emphasising the importance of tailored strategies to ensure therapeutic efficacy and safety.1
DEVICES FOR RESPIRATORY DELIVERY – A KEY CHOICE
The successful delivery of inhaled biological drugs requires not only a specific formulation but also a device to generate aerosols. Due to the complex structure of biologics, the aerosolisation process needs to produce particles with aerodynamic properties suitable for inhalation and preserve the physical integrity and potency of the molecules at the same time.
Therefore, the selection of the appropriate device is critical as each device offers distinct advantages and disadvantages in terms of key performance parameters, which include precise and reproducible dosing, efficient delivery of high doses with minimal losses and a favourable lung deposition profile.
Given that approximately 75% of protein formulations intended for inhalation in clinical research are in liquid form,2 nebulisers – including jet, ultrasonic and vibrating mesh – are the most used devices for administering biologics to the lungs. This relates to their high efficiency, possibility of targeted lung deposition and compatibility with liquid formulations.1
For patients, nebulisers are generally suitable for all ages and disease stages, accommodating different inspiration flow rates and breathing capacities. However, the general process of nebulisation exposes the proteins to physical stress, such as shear forces and heat. Additionally, the large air:liquid interface can alter protein conformation and structure, which is particularly relevant with jet and ultrasonic nebulisers.
By comparison, vibrating mesh nebulisers (VMNs) use a vibrating element connected to a mesh to generate aerosols in a single-pass process. This minimises the temperature increase and the shear stress that the molecule is subjected to, which is crucial for preserving the biological activity of macromolecules as biologics.3 Moreover, compared with other types of nebulisers, VMNs have increased nebulisation efficiency – higher drug delivery in less time – making them the preferred choice for the nebulisation of proteins.1
NEBULISING BIOLOGICS – CHALLENGES AND CONSIDERATIONS
The inhalation of biologics is not a straightforward process as there are several challenges to overcome:
- The natural protective mechanisms of the lung
- The pharmaceutical properties of the formulation related to biologics delivery, such as protein stability, aggregation and the consequent potential for immunogenicity
- The interaction between the device and the drug, ultimately influencing its availability in the lungs.
Understanding and acknowledging these challenges is crucial for mitigating the risks associated with respiratory delivery of biologic drugs. The drug and the device should be optimised together to achieve a desirable aerosol performance and retain the molecular integrity of biologics.
Optimising lung delivery for biologics requires early strategies in preclinical development to tailor specific nebuliser features to refine device and formulation according to each target and indication. By effectively delivering drugs directly to the site of action within the lungs, aiming for either local or systemic delivery, higher intended drug concentrations are achieved, while reducing losses and exposure in non-desired areas. This is particularly relevant for biologics, which are usually expensive drugs. Using a nebuliser, targeted drug delivery can be achieved through the intentional adjustment of influential parameters on lung deposition, including the breathing manoeuvre and the aerosol characteristics within the patient’s respiratory system. Existing devices, such as the Kolibri mesh nebuliser, use innovative inhalation feedback technology to guide the patient’s inspiratory manoeuvre and an aerosol generator tailored to each specific drug product. Ultimately, this approach requires selecting the right nebuliser for maximising therapeutic efficacy while minimising risks.
CURRENT RESEARCH
There have been significant advances in aerosol delivery technologies in the last two decades since the approval of two of the most relevant examples of inhaled biologics: inhaled insulin and dornase alfa.
The systemic delivery of biologics through the lungs is exemplified by inhaled insulin in dry powder. Despite being a global breakthrough in biotherapeutics, the first marketed inhaled insulins faced challenges due to issues with dosing, low device acceptability and portability, leading to low sales and their withdrawal soon after. These drawbacks diminished the market interest and since then only one other dry powder insulin was approved in 2014, with improved formulation technology, dosing flexibility and a much more portable device.
Dornase alfa, an inhaled recombinant human deoxyribonuclease I (rhDNase) for the management of cystic fibrosis, was initially approved only for jet nebuliser/air compressor combinations but later also for a VMN, considering the advantages related to higher efficiency, shorter treatment times and patient preference.
These lessons highlight the importance of technological advancements in formulation and device, as well as patient acceptance in inhaled biologic product success.
Currently, several inhaled biologic formulations for different respiratory and systemic disorders are advancing through clinical development. Liang et al (2020) provide a comprehensive table with a summary of the clinical trials with inhaled biologics and the chosen device.4 Most of the listed programmes are with nebulisers, and many clearly state the use of VMNs. The majority target respiratory diseases, such as chronic obstructive pulmonary disease, cystic fibrosis, respiratory viral infections and asthma, but also systemic disorders, such as diabetes and anaemia.
FUTURE DIRECTIONS
Future advancements for inhaled biologics hold promise for enhanced therapeutic outcomes. Research is currently focused on refining drug characteristics to improve the delivery of biologics, addressing challenges such as molecular stability, lung absorption and aerosol performance of biologics formulations. For an optimal aerosolisation process, focus on the device is key. Nebulisers that allow customisation and adjustment to each specific biologic drug can represent a future advantage, as well as other important patient-centric features, including inhalation guidance technology and connectivity for user and clinical development support. These initiatives underscore the evolving panorama of nebulised biologics, paving the way for personalised and more effective treatments in the future.
REFERENCES
- Froehlich E, Salar-Behzadi S, “Oral inhalation for delivery of proteins and peptides to the lungs”. Eur J Pharm Biopharm, 2021, Vol 163, pp 198–211.
- Bodier-Montagutelli E et al, “Designing inhaled protein therapeutics for topical lung delivery: what are the next steps?”. Expert Opin Drug Deliv, 2018, Vol 15(8), pp 729–736.
- Matthews AA, Ee PLR, Ge R, “Developing inhaled protein therapeutics for lung diseases”. Mol Biomed, 2020, Vol 1(1), p 11.
- Liang W et al, “Pulmonary Delivery of Biological Drugs”. Pharmaceutics. 2020, Vol 12(11), p 1025.

