To Issue 133
Citation: Gross C, Romańczuk R, “Improving Clinical Outcomes with a Patient-Centric and Reusable Pen Platform”. ONdrugDelivery, Issue 133 (May 2022), pp 70–74.
Cécile Gross and Radosław Romańczuk discuss Nemera’s Pendura AD pen platform and present case studies demonstrating how it can be tailored to address the user’s needs and meet pharma industry constraints.
“Benefiting from 10 years of experience and market presence, Nemera’s Pendura AD platform encompasses all the features needed to comply with any requirement.”
The year 2022 celebrates the 100th anniversary of the first insulin injection performed on a human being, a 14-year-old boy suffering from Type 1 diabetes. This ground breaking scientific and clinical achievement occurred in Canada, although the disease had been identified by Aretaeus, a disciple of Hippocrates, around 100 AD. Since then, diabetes has become a chronic disease classified by the WHO as one of the four main non-communicable diseases (NCD).1 So, have we stopped in midstream? Of course not. There have been numerous changes and evolutions in therapies and in injection.
One main revolution is the drug itself: patients now have access to human as well as analogue drugs. Another revolution is related to drug delivery devices: we have turned the corner from simple glass syringes to safe, self-administered injection devices. Both customer and patient requirements have forced manufacturers to improve their technology and provide best-in-class devices.
Nemera is no exception. The Pendura AD platform provides a multiple-use reusable pen injector that can address both the user needs and the pharma industry constraints (Figure 1).

Figure 1: Pendura AD’s key features designed to optimise the patient experience.
Benefiting from 10 years of experience and market presence, Nemera’s Pendura AD platform encompasses all the features needed to comply with any requirement. From the beginning of the device conception, the context of growing prevalence of chronic diseases has been central. The burden of lifelong treatment is cumbersome for patients, and treatment adherence becomes crucial in order to ensure clinical outcomes. For example, several acceptability studies have been conducted focusing on insulin-naïve individuals.2,3 The learning curve is also at stake; it goes without saying that psychological considerations must not be underestimated. Overall, it is a question of accuracy, limited pain and discretion.
So, how does this translate into device features? First, of course, is the injection mechanism: a spring-driven movement ensures drug delivery is automatic and results in a smooth injection. There is, therefore, no need to apply manual force to perform the injection. Besides, this movement is easily triggered by a side button, which allows the user’s hand to be stabilised during the injection process.4 Second is dose checking: the cartridge containing the drug is inserted into a transparent cartridge holder, where visual inspection of the correct mixture can be performed. By turning the dose knob with audible clicks, the user can adjust the dose, and the dose selection window clearly shows the dose in black numbers on a white background. One significant advantage is that correcting the selected dose does not result in losing drug. To be sure that the full dose has been delivered and the pen is not removed before completion, a coloured dot is made visible close to the dose knob and the thumb positioned on the side activation button. Using a new cartridge implies priming, which is also easy to perform.
“It has also been proven that using a pen device for insulin therapy
improves adherence, enhances quality of life, reduces the risk of hyperglycaemia and decreases costs.”
FROM A HEALTHCARE PROFESSIONAL PERSPECTIVE
This article has discussed how Nemera’s Pendura AD platform has been conceived from the patient’s perspective, but healthcare professionals have not been forgotten. Even if the device is a self-administered device, these professionals are heavily involved in educating patients.5 They are first to deal with adverse clinical outcomes; for them, the device must be easy to learn, easy to prepare and easy to administer. Nurses, as healthcare professionals on the frontline, allocate a great deal of attention to training time. Because their own caring time per patient is limited, so is training time. Simplicity of use is mandatory and a key concern, as a complex-to-use or non-reassuring device inevitably results in longer training or the device will, in the end, not be used at all. In the same way, the combination drug and device are part of the overall treatment protocol. If using the drug delivery device may jeopardise the clinical outcomes of the treatment, the device will not be demonstrated, recommended or prescribed. So, the same device features also provide benefits for this category of stakeholders.
Let’s now have a closer look at the currently marketed applications: insulin, peptide, parathyroid hormones and human growth hormones, all of which require daily subcutaneous injections (Figure 2).
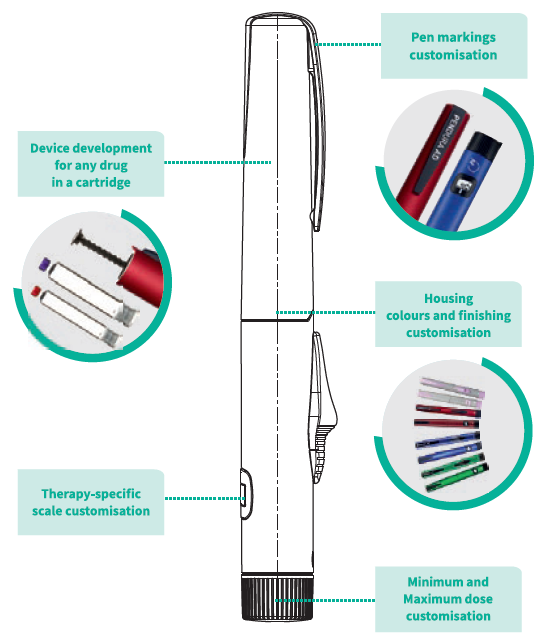
Figure 2: Highly customisable platform to fit pharmaceutical and patient needs.
CASE STUDIES
Case Study One: Insulin and its Analogues
Thanks to Banting and Macleod who received the Nobel Prize for its discovery, insulin has been used for a long time, and insulin therapy is now well documented, especially from the drug delivery device perspective. As already mentioned, delivery devices have evolved significantly since glass vials. It has also been proven that using a pen device for insulin therapy improves adherence, enhances quality of life, reduces the risk of hyperglycaemia and decreases costs. Using a reusable pen device for such therapy includes extra benefits, such as durability and flexibility; storage and carrying the mandatory doses for several days becomes easy as well. In addition, the dial-back possibility prevents loss of insulin and changing the cartridge is easy to perform.
Nemera’s first partner in this field performed a series of post-market studies that showed Pendura AD was perceived as “comfortable” to use and pain severity and discomfort decreased during the assessment period.6 A significant reduction in glycaemic level quantified by HbA1c measurements was also noted – daily-life improvement was assessed through an increase of the HRQoL score.7
Along with the evolution of drugs is the possibility of producing molecules identical to human ones. A good example is glargine insulin. Modifying the order of amino acids enables control of how the molecule is incorporated in the body. A small amount of the material moves into a solution in the bloodstream, while the remainder is sequestered in subcutaneous tissue for up to 24 hours, which is the reason why it is classified as a long-acting insulin. Other types of insulin are combined with short-acting insulins as part of a drug regimen targeting glycaemic control.
Biomm (São Paulo), a pioneer in Brazilian biotech/pharma with a facility of 12,000 square metres, combined the two, creating an innovative drug and delivery device, by offering a reusable pen for its first ANVISA (Brazilian Health Regulatory Agency) registered glargine insulin – a 3 mL cartridge containing 100 IU of drug per mL. After some technical adjustments of Nemera’s technology to fit Biomm’s needs, Lifepen G is now marketed, offering 60 increments and a pleasant outer shape in a grey and purple colour combination, which is a smart way to avoid confusion between insulin types.
Case Study Two: Glucagon and its Analogues
Although insulin is produced by the beta cells of the pancreas, glucagon is produced by the alpha cells. In short, whereas insulin decreases the blood sugar level, glucagon increases it. Twins, but “sister enemies”. This component of pancreatic extract was identified back in 1922 and named after a portmanteau of “glucose agonist”. Its properties have been fully understood since the 1970s, and it has followed the same path as its “sister” and is now available in a synthetic form.
Nemera’s partner Zealand Pharma (Copenhagen, Denmark) is offering dasiglucagon, a next-generation ready-to-use glucagon analogue. Dasiglucagon is being developed for several therapies. Thanks to a constructive co-operation between the two entities, the Pendura AD platform has been selected to deliver dasiglucagon, and this combination is being investigated in clinical trials.
Case Study Three: Parathyroid Hormones and its Analogues
Teriparatide is a portion of human parathyroid hormone, a primary regulator of calcium and phosphate metabolism in bone and kidney. Aimed at treating osteoporosis, its intended use is for both men and postmenopausal women who are at risk of bone fracture. In the same way as insulin previously, it was first approved by the US FDA 20 years ago and has been extended now to biosimilar, synthetic and recombinant versions.
At stake here is the risk of fracture, which, for the patient, means input from healthcare professionals, hospital stays, rehabilitation, potential complications, etc. Several clinical studies have shown real benefits, such as an increase in both bone mineral density and bone mineral content. However, as the drug is approved for a total treatment duration limited to 24 months, the patient journey rather resembles a speed race, and treatment adherence is crucial.8
To secure the use of Pendura AD in this application, Nemera’s partner, a leading multinational pharmaceutical company, has performed a human factors engineering study, which led to the conclusion that this reusable pen device was the solution for achieving the best clinical outcomes for patients. This choice has been confirmed with another partner, Enzene Biosciences (Pune, India), whose core values are enabling novel therapies.
Case Study Four: Human Growth Hormones (HGH)
Another therapeutic area is growth hormone deficiency (GHD) and other growth-related syndromes treated with growth hormones, which have switched in the past decade from pituitary-derived human to biosynthetic and recombinant options. At first glance, this should be restricted to paediatric treatment only, but adults as well as children can be affected. For children with GHD, therapy would be from the age of diagnosis to early adulthood, while for adults with GHD, therapy may be for life. Needless to say, adherence is key.9,10

Figure 3: In-house manufacturing capability with high flexibility for production-scale adaptation.
Scigen (IL, US), an integrated pharmaceutical company engaged in research and development, production and commercialisation of API and finished dosage forms, was looking for a reliable reusable pen for its somatropin. Three types of doses of SciTropin A™ (somatropin injection) are available: 2, 4 and 6 mg in cartridges of 1.5 mL. Three pens are now marketed with three different references (5, 10 and 15) and three different colours (blue, green and burgundy).11
Ultimately, what does it truly mean to Nemera to offer a patient-centric and reusable pen platform? It means offering the company’s partners the possibility to use this reliable platform, to adapt it to their needs and to optimise the patient experience, eventually (Figure 3).
In addition to the basic features already described and tangible examples of market presence, Nemera can provide support at every step of the process, from technical adjustments according to cartridges and doses, device customisation, small-series manufacturing, clinical-batch manufacturing, high-scale manufacturing, regulatory approval and market launch.
Willing to expand its capabilities, a new plant is under construction in Poland. Gathering state-of-the-art equipment from moulding to assembly and quality control testing, this brand-new facility will be ready to operate in the fourth quarter of 2022 (Figure 4).

Figure 4: Extending Nemera’s manufacturing capabilities with a new state-of-the-art facility in Szczecin, Poland.
With 22,500m² of building overall, including an ISO 8 clean room, this digitally native plant is to become carbon neutral. Implementing BREEAM recommendations for this construction is one way to fulfil Nemera’s commitment to sustainability as a company (Figure 5).12

Figure 5: Custom and agile services to support pen injector projects holistically.
A wide range of services is also available throughout the device lifecycle, in every development phase. From device strategy Nemera Figure 4: Extending Nemera’s manufacturing capabilities with a new state-of-the-art facility in Szczecin, Poland. |and development, clinical trials and industrialisation to lifecycle management, Nemera’s experts can help with the patient journey, human factors and user experience and regulatory dossier submission, to name but a few.
With proven records of patient acceptance and partner satisfaction, the Pendura AD reusable pen platform is robust, flexible and customisable, dedicated to optimising clinical outcomes for patients with critical long-term treatments. This platform, offering holistically state-of-the-art capabilities and services to pharma partners, is at the heart of Nemera’s business.
REFERENCES
- “Noncommunicable diseases”. WHO, Apr 13, 2021.
- Tschiedel B et al, “Initial Experience and Evaluation of Reusable Insulin Pen Devices Among Patients with Diabetes in Emerging Countries”. Diabetes Therapy, 2014, Vol 5, pp 545–555.
- Wong M et al, “A randomized, cross-over comparison of preference between two reusable insulin pen devices in pen-naïve adults with diabetes”. Curr Med Res Opin, 2013, Vol 29(5), pp 465–473.
- Fendler W et al, “Trigger matters: an ergonomy analysis of insulin pens”. Diabetes Technol Ther, 2015, Vol 17(3), pp 171–176.
- Zitoun P et al, “Analysis of patient and nurse preferences for self-administered FSH injection devices in select European markets”. Int J Womens Health, 2019, Vol 7(11), pp 11–21.
- Gorska-Ciebiadaa M, Masierekb M, Ciebiadac M, “Improved insulin injection technique, treatment satisfaction and glycemic control: Results from a large cohort education study”. J Clin Transl Endocrinol, 2020, Vol 19, 100217.
- “Health-Related Quality of Life (HRQOL)”. Centers for Disease Control and Prevention, Jun 16, 2021.
- Lindsay R et al, “Teriparatide for osteoporosis: importance of the full course”. Osteoporos Int, 2016, Vol 27(8), pp 2395–2410.
- Sauer M, Abbotts C, “A new pen device for injection of recombinant human growth hormone: a convenience, functionality and usability evaluation study”. Patient Prefer Adherence, 2017, Vol 27(12), pp 27–234.
- Cachemaille A, Warren SE, Moss S, “A pen device for injection of recombinant human growth hormone: a European usability engineering study”. Expert Opin Drug Deliv, 2020, Vol 17(7), pp 1041–1048.
- “SciGen receives approval from Australian Register of Therapeutic Goods, for Nemera’s reusable pen injector, for its human growth hormone”. Press release, Nemera, March 17, 2022.
- BREEAM Sustainability Assessment Method. www.breeam.com, accessed Apr 2022.

