Citation: Sarkar M, Samaranayake I, “Improving Patient Outcomes with Advanced Digital Biomarkers”. ONdrugDelivery, Issue 121 (Jun 2021), pp 27–30.
Matthew Sarkar and Imantha Samaranayake, consider the contribution digital biomarker devices make towards healthcare and the challenges faced by the evolution of such devices.
Digital biomarker devices could bring about a new age of intelligent, personalised treatments and care plans that significantly improve healthcare effectiveness and cost-efficiency. However, to make a meaningful difference to patient experiences and outcomes, they need to do more than simply monitor an individual’s condition. They also need to analyse and leverage biomarker data to generate actionable insights that can inform decisions as patients progress along treatment plans.
“In a world reshaped by covid-19, perhaps one of the most noteworthy opportunities related to digital biomarkers is the ability to monitor, and thereby manage, disease outbreaks. For instance, digital biomarkers might be used to track indicators such as oxygen saturation, temperature, respiratory rate and voice characteristics.”
As the technology that underpins digital biomarker devices becomes more advanced, exciting new capabilities are within reach. However, the development of digitally enabled medical devices is rarely straightforward. To gain regulatory approval, they need to satisfy stringent safety and security measures that go beyond the safety of treatment itself. This introduces an additional layer of complexity to the product development journey. Many practical and technical factors can impact effectiveness and uptake as well.
Innovation teams developing solutions using digital biomarkers need to consider the role they will play in wider digital therapeutics as well as technical and regulatory matters. Making time for this at the earliest possible stage in product development is crucial. When decisions are shaped by an awareness of the bigger picture, it makes for a smoother and faster product development journey.
WHAT ARE DIGITAL BIOMARKERS?
Digital biomarkers are quantifiable, measurable health indicators that can be collected and analysed in the cloud or, as capability improves, at the network edge. Comparing data gathered at the individual level with big data from wider cohorts or populations can help explain, influence or predict health-related outcomes. Devices incorporating digital biomarker technology can be implemented in different formats, such as wearable, implantable, or even ingestible.1
Figure 1 shows various digital biomarker types across six categories (vital signs, physical, chemical, neural, visual and audible). For more detail on each category, such as application areas and biomarker formats, there is an extended, interactive version of the diagram available on Sagentia’s website, here. This online resource also demonstrates the value of using multiple digital biomarkers in the detection and management of certain conditions.
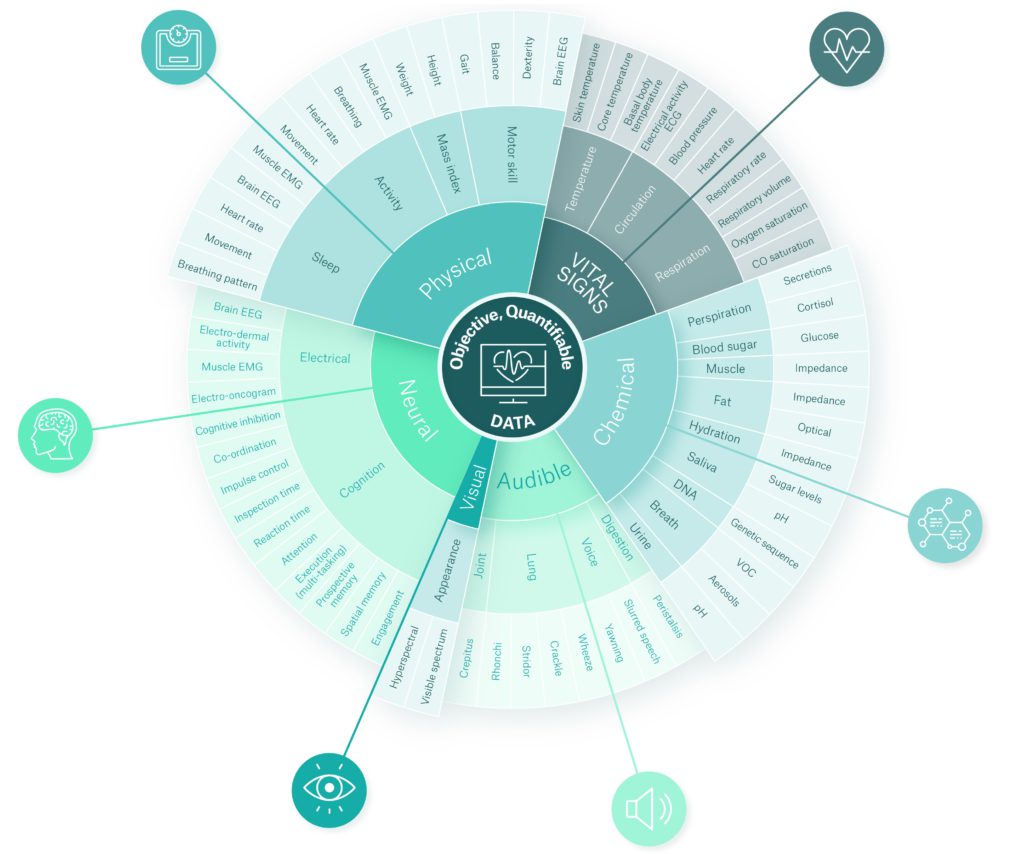
Figure 1: Six digital biomarker categories with physiological and behavioural data. An extended, interactive version of the diagram is available on Sagentia’s website, here.
“With the creation of biomarker “profiles”, there could be a step-change in general diagnostics for many, if not all, illnesses and conditions.”
OPPORTUNITIES IN THE DIGITAL BIOMARKER SPACE
Disease Management
In a world reshaped by covid-19, perhaps one of the most noteworthy opportunities related to digital biomarkers is the ability to monitor, and thereby manage, disease outbreaks. For instance, digital biomarkers might be used to track indicators such as oxygen saturation, temperature, respiratory rate and voice characteristics. This could happen in discrete hospital settings, the care home network or even within the larger population of patients with the illness, or people at high risk.
Such developments could drive benefits at a global, national, local or individual level. They might provide early warning of infection, triggering self-isolation, or be used to enable rapid identification of illness clusters by predicting areas where hospital resources are likely to come under pressure. At a larger scale, data could be used to generate insights on the disease symptom trajectory. This could inform trials for treatments and vaccines or enable national and global surveillance.
Risk Stratification
Monitoring digital biomarkers throughout disease progression unlocks exciting opportunities. It is possible to gain a deeper understanding of illnesses and conditions and then stratify potential outcomes for different patients, offering new ways to personalise treatment. Detailed studies on the rates of change for certain biomarkers over time could offer valuable insights too.
Take increased body temperature – in general terms, this signifies a viral infection. But does the shape of a certain temperature profile over a matter of hours or days signify a particular infection or condition? It may be possible to establish longitudinal profiles for seasonal flu viruses or SARS-CoV-2, for instance.
When looking beyond an individual episode of care, the potential is even greater. With the creation of biomarker “profiles”, there could be a step-change in general diagnostics form any, if not all, illnesses and conditions. Does the way a particular biomarker changes over time suggest a particular illness? It could certainly narrow down the range of likely illnesses, providing early indication on a much broader scale than current capabilities allow, and facilitating a more targeted and precise diagnosis. Combining longitudinal profiling with the ability to cross-compare and blend a multitude of biomarkers would drive significant progress.
Long-Term Care
“Devices need to operate within existing regulatory parameters while being future-proofed for potential changes. Monitoring current developments beyond the medical sector can help inform decisions related to this.”
The treatment of chronic conditions, especially in the context of ageing populations, can be greatly enhanced with the use of digital biomarker devices. Continuous monitoring provides opportunities for earlier intervention, an objective overview of disease management effectiveness or early warning signs if a condition deteriorates. This potentially goes beyond current goals of rapid intervention at the onset of exacerbation, to true early intervention avoiding the exacerbation altogether.
Advantages for both patient health and health system economics are enormous. For example, if a COPD patient is alerted early, takes preventative action and avoids a hospital stay. Conducting this by remote patient management is potentially cheaper, avoids the risk of infection in healthcare environments and is more convenient for people experiencing pain or mobility issues. More than 15 million people are living with chronic conditions and require long-term disease management in the UK alone. And many countries are facing the challenge of caring for ageing populations. In this context, digital biomarker devices could play a significant and valuable role in improving patients’ quality of life while reducing the burden on healthcare systems.
Mental Health Conditions
The increased incidence, and awareness of, mental health issues presents another avenue where digital biomarker devices could vastly improve patient outcomes and quality of life. Such devices can act as a constant companion, monitoring conditions in real-time and then triggering interventions at critical moments prior to the onset of a significant event. They can promote activities to help counter or prevent negative or destructive thoughts, and provide access to supportive communities.
Personalised Preventative Care
Early intervention to prevent or delay the onset of chronic conditions is a prime area for the next generation of digital biomarker devices. For instance, tracking an individual’s weight and glucose levels could aid the prediction and prevention of Type 2 diabetes. This concept of personalised, preventative healthcare linked to digital biomarker devices is gaining a lot of attention. For instance, in the US it has the potential to drive more sophisticated approaches to healthcare provision, such as personalised insurance premiums and value-based treatment.
The Digital Health Act (DVG), which took effect in Germany in 2020, looks set to drive increased use of digital biomarkers. Under the DVG, doctors or psychotherapists will be able to prescribe low-risk digital health apps where efficacy has been proven to the Federal Institute for Drugs and Medical Devices (BfArM). This is part of a range of measures geared towards expanding the digitalisation of Germany’s health services. Regulatory requirements for DVG apps include privacy law compliance and a high level of data security. Nevertheless, patient advocacy groups have raised concerns surrounding the inability to opt-out of anonymised data sharing for research purposes. This situation underlines the complexities facing digital biomarker device development.
CHALLENGES IN DIGITAL BIOMARKER DEVICE DEVELOPMENT
Many of the barriers to digital biomarker device approval, effectiveness and uptake, relate to the use and management of patient data. Much of the time, there is a lag between standards or regulations and emerging technical capabilities. So, devices need to operate within existing regulatory parameters while being future-proofed for potential changes. Monitoring current developments beyond the medical sector can help inform decisions related to this.
Another challenging area that can hinder device approval is the need for continuous modification of the algorithms related to digital biomarkers. Again, careful consideration at an early stage in device development is the best solution. In this way, it is possible to maintain substantial equivalence from the point that the device is approved.
Finally, since digital biomarkers are generally proxy measurements for disease conditions, rigorous validation of what they indicate is essential. However, intellectual property concerns and the nature of compiled code can lead to a lack of transparency, which makes independent scrutiny a challenge. Machine-learned algorithms further obscure underlying mechanisms as they learn and adapt according to the data provided.
Data Privacy, Quality, Management and Analysis
For digital biomarkers to drive effective medical treatments and practices, they need to draw on large datasets. Yet, there are many factors that hinder this in practice. Challenges range from data privacy laws – and disparities in those laws between different countries – to the storage, analysis and standardisation of patient information. In addition, electronic medical records are currently not designed to store large amounts of continuous data or to conduct real-time analytics. Data quality is an issue as well. Variations between devices, and in the ways people use or wear them, can lead to significant differences in data collected. This can skew analysis and anomalies may not be easy to distinguish.
Lack of standardisation between healthcare settings and countries can also impede the value of digital biomarker data. As data is collected and controlled by separate entities, silos inevitably arise. This limits data liquidity preventing its flow through the wider healthcare system.
The Need for Standardisation
All the above factors impact the collection, analysis and value of digital biomarker data. So, there is a pressing need to achieve better standardisation of data storage, labelling and tagging.
This is partly about improving the ease with which information can be processed and exchanged. But more importantly, it is about providing context for each datapoint to drive deeper levels of insight that enhance understanding at the macro-level while driving better patient experiences at the individual level.
New frameworks are emerging to address some of the challenges surrounding security, ethics and informed consent in digital phenotyping. For instance, the US FDA’s proposed Cybersecurity Bill of Materials for the premarket submission of medical software would aim to identify issues related to security vulnerabilities, transparency and accuracy.
Digital biomarkers are inherently changeable, and the understanding of the way they signpost conditions evolves over time as more data are gathered. However, patient consent tends to relate to specific, predefined scenarios. So, the ability to update consent easily and conveniently on a regular basis can be a critical success factor in the regulatory approval of devices.
Room for Improvement
While many of the technologies that underpin digital biomarker devices are maturing, there are some serious issues that need to be addressed. For instance, when users from different ethnic backgrounds interact with devices the results must be consistently reliable. However, this is not always the case. Facial recognition software tends to deliver higher levels of accuracy for light-skinned users than for darker-skinned users. Issues like this need to be anticipated and resolved at an early stage in product development. It is sobering to recognise that an “unbiased” algorithm will machine learn the unconscious bias of a coder who provides it with a skewed data set. Without scrutiny at a fundamental dataset level, this ghost in the machine could proliferate at an alarming rate, obscured by machine-learned code and scaled through digital deployment.
THE WAY FORWARD
In recent times, we have seen a convergence of technical capabilities, healthcare industry needs and wider acceptance of remote, digitally enabled medical treatment. Together, these factors indicate that the market is calling out for innovative medical devices rooted in digital biomarkers. Additionally, as digital biomarker devices evolve to offer more robust, actionable, predictive insights, they are set to play a fundamental role in the wider digital therapeutics space.
Challenges do exist, and they are complex. But they are not insurmountable. At Sagentia Innovation, scientists and technology specialists with extensive experience in the medical sector work collaboratively to solve problems of this nature. When potential barriers to approval or adoption are properly accounted for early in the design phase, the underlying software can be adapted to accommodate or mitigate them.
Digital biomarkers are transforming our understanding of diseases and chronic health conditions. As the devices measuring them progress to offer predictive analytics, which enables early intervention, we will see a revolution in treatment protocols. Over time, this will drive better patient experiences and outcomes while making healthcare provision more efficient, effective and individualised. Medical device manufacturers that act now will be in a strong position to play a lead role in this new age of healthcare.
REFERENCES
- Babrak L M et al, “Traditional and Digital Biomarkers: Two Worlds Apart?”. Digital Biomarkers, 2019, Vol3(2), pp 92–102.

