To Issue 176
Citation: Schmidt P, “Injecting Drugs Directly into the Body: How On-Body Delivery Systems are Revolutionising Therapy”. ONdrugDelivery, Issue 176 (Sep 2025), pp 24–26.
Peter Schmidt discusses the advantages of on-body delivery systems and what makes them special, going on to consider the significant role that they are expected to play in the future of injection technology for the pharmaceutical and medical fields, as well as the role that comprehensive testing plays in bringing this technology to patients.
Medicine is becoming portable – while going to the clinic used to be routine for many patients with chronic illnesses, modern on-body delivery systems (OBDSs) can now enable painless and discreet self- administration of medications via a device worn directly on the body, in the patient’s own home. This has long been a reality and has not only changed the quality of life of those affected, but also the processes in the healthcare system.
WHAT MAKES OBDS SO SPECIAL
OBDSs are portable injection systems that are attached to the skin and administer medication over a longer period of time in a controlled, precise and virtually unnoticed manner. These devices offer a real alternative to traditional administration methods, particularly for highly viscous biologics, which are difficult to administer using conventional autoinjectors.
Biologics – usually proteins – often must be delivered via an injection, as they would be broken down in the gastrointestinal (GI) tract. Biologically active molecules often have a large, complex and fragile structure, which makes them susceptible to degradation in the body. Their low permeability through biological barriers and complicated transport to the intracellular target make it difficult for them to maintain efficacy. However, an injection can significantly increase the bioavailability of these molecules, as it allows direct access to the bloodstream, bypassing degradation in the GI tract.
Treatment with OBDSs can be administered alone at home – without the need for a healthcare professional. This means that patients have to go to hospital less often, which places less of a burden on healthcare systems. OBDSs are easy to use, meaning that more people adhere to their treatment and digital aids, such as apps, make it easy to monitor treatment. Delivery via an OBDS can also save money because it reduces the necessary number of clinical appointments for infusion. As such, OBDSs enable a new form of patient autonomy, giving people back their time and freedom, while maintaining a consistently high level of therapeutic safety.
A GROWING MARKET WITH COMPLEX REQUIREMENTS
The market for OBDSs is growing rapidly. More and more companies are investing in the development of new generations of devices that are not only functional but also user-friendly and aesthetically pleasing. The variety of models – ranging from disposable to reusable devices, as well as offering different drug reservoirs and drive technologies – presents developers and test engineers with new challenges. A key issue is the reliability of drug delivery; with highly viscous substances in particular, it is crucial that the entire volume is injected correctly – right to the last drop. Simultaneously, these devices must be tested under realistic conditions, such as at body temperature. As a specific example, cytostatic drugs are used in cancer therapies, but they are hazardous to health if inhaled, therefore, to counteract this hazard, ZwickRoell has already installed devices for air extraction as part of its testing technology.
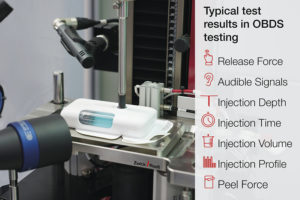
Figure 1: Typical test values that are preferably determined in a single test run.
TESTING EXPERTISE FOR THE NEXT GENERATION OF INJECTION TECHNOLOGY
As one of the leading suppliers in the field of testing technology, ZwickRoell, together with a well-known manufacturer of injection systems, has developed an innovative testing system for OBDSs. This system now makes it possible to perform all relevant tests in a single pass – efficiently, modularly and adaptably to different injector types (Figure 1). A particular highlight is the option to combine several testing tasks, such as peeling off the adhesive film and injection testing (Figure 2), in one machine. This saves time and resources and increases the validity of the tests. ZwickRoell’s testing systems are designed so that they can be flexibly adapted to new requirements, which is crucial, since the variety of OBDS models is constantly growing.

Figure 2: Peel forces for the film are determined in the peel test.
FROM THE INITIAL IDEA TO QUALIFICATION – EVERYTHING FROM A SINGLE SOURCE
ZwickRoell supports its customers throughout the entire project, from the initial user requirement specification (URS) to the final qualification. Standardised checklists, URS templates for all common injection systems, a standardised process and an experienced project team ensure that no detail is overlooked. The company’s own qualification department offers both standardised and customer-specific solutions, including on-site measurement system analyses.
ZwickRoell’s range of testing machines is supplemented by testXpert III testing software with integrated user administration. Complete traceability and tamper-proofing in accordance with US FDA 21 CFR Part 11 is made possible by an optional integrated software module. The integrity of data cannot be negotiable, as it is the only way to guarantee the security of results and their traceability, as well as provide protection against manipulation.
“THE FUTURE OF OBDSs LIES NOT ONLY IN THE MECHANICS OF THE DEVICES, BUT ALSO IN DIGITAL NETWORKING, VERSATILITY AND PATIENT COMFORT.”
DIGITAL NETWORKING IS THE FUTURE
The future of OBDSs lies not only in the mechanics of the devices, but also in digital networking, versatility and patient comfort. The results of injections and raw data can be precisely documented using connectivity – more and more systems are equipped with sensors that document the injection process, communicate with apps or even transmit real-time data to medical professionals. This development opens up new possibilities for personalised therapies and close monitoring, especially for cancer and chronic diseases. ZwickRoell takes this development into account by also providing connectivity and data management solutions. This means that not only mechanical but also functional and software-based aspects of OBDS can be reliably tested.
REGULATORY REQUIREMENTS AND FUTURE PROSPECTS
As technology advances, so do the regulatory requirements for OBDSs. Authorities such as the FDA and EMA not only require proof of mechanical safety but also functional reliability and data integrity. Compliance with standards such as ISO 11608-5 and ISO 11608-6 is an essential part of this, especially when digital components are integrated. ZwickRoell supports manufacturers in implementing these requirements with validated test processes, documented testing software and comprehensive qualification services.
“NEW MATERIALS, MINIATURISED ELECTRONICS AND AI-SUPPORTED EVALUATION SYSTEMS ARE SET TO OPEN UP COMPLETELY NEW POSSIBILITIES.”
Looking into the future, it seems inevitable that OBDSs will continue to gain in importance. New materials, miniaturised electronics and artificial intelligence (AI)-supported evaluation systems are set to open up completely new possibilities. For example, systems that automatically recognise the optimal injection time or adapt the administration of medication to individual vital parameters are conceivable.
The industry is only at the beginning of a development that will permanently change the healthcare sector. The combination of medical technology, digitalisation and patient focus will produce many new solutions in the coming years – and ZwickRoell is ready to help shape them.
OBDSs ARE MORE THAN JUST A TREND
OBDSs are exemplary for the change in medical technology away from centralised, clinic-based therapies towards patient-centric, digital and flexible solutions. They not only improve quality of life for patients but also the efficiency of healthcare systems. The integration of smart wearables into medication administration opens up new avenues for patient-centric care. The combination of digital monitoring, automated dosing and AI-supported analysis promises not only greater adherence to treatment but also more efficient use of medical resources. With innovative testing systems, standard-compliant implementation and comprehensive project support, ZwickRoell is making an important and decisive contribution to improving the quality and safety of this new technology – and thus to the future of drug delivery.

