To Issue 171
Citation: “Interview with Patrick Anquetil. A Sustainable Future for Self-Injection with Connected, Reusable Autoinjectors”. ONdrugDelivery, Issue 171 (Apr/May 2025), pp 52–55.
In this exclusive interview with ONdrugDelivery’s Guy Furness, Dr Patrick Anquetil, Chief Executive Officer of Portal Instruments, offers insight into the extensive waste involved in the self-injection market. He explains how pivoting from a model focused on fully disposable devices to reusable electromechanical autoinjectors could offer a more sustainable and cost-effective path forward. Dr Anquetil also introduces PRIME Nexus, Portal’s adaptation of its flagship needle-free PRIME device to a scaled-down, needle-based alternative.
Q Before we delve into sustainability, could you give our readers a quick overview of Portal Instruments and its platform products?
A Portal Instruments was started about a decade ago, with a broad idea to change what it means to self-inject, as well as to change injections as a whole. Our founding principles start with Ian Hunter, a professor from the Massachusetts Institute of Technology (Cambridge, MA, US), who brought me in to lead Portal as a start-up. From the start, our approach has been to look at injections at the system level so, at heart, we’re an injection technology company, our flagship product being the PRIME needle-free system.
What we realised with PRIME is that, while patients prefer needle-free injections and its premium features, there is also a need for a lower cost solution. So we set out to create a device that is in-between a needle-free injector and an autoinjector, but that still uses the same platform and is able to use the scaling effect that an electromechanical system can bring. This led us to simplify and miniaturise our existing needle-free platform while making it compatible with all standard primary containers used in the pharma industry, such as prefilled syringes or cartridges. That’s what PRIME Nexus is – a flexible, needle-based variation of our PRIME platform (Figure 1).

Figure 1: Portal’s PRIME Nexus reusable autoinjector.
“ALL OF PRIME’S ADVANTAGES AND ALL THE TECHNICAL KNOW-HOW THAT WE’VE DEVELOPED AT PORTAL OVER THE LAST DECADE EASILY TRANSLATES TO NEXUS.”
We have been working on the core electromechanical platform for a long time, so we’ve been able to bring all of its advantages – programmability, high precision, rapid adjustability for viscosity and volume – to a new product without having to go through a whole new, ground-up development process. All of PRIME’s advantages and all the technical know-how that we’ve developed at Portal over the last decade easily translates to Nexus.
Q Beginning with a general question on sustainability – why is it that the pharma industry should be concerned about sustainability?
A I think the number one thing is cost. Single-use autoinjectors are amazing devices; they’re produced in very high numbers with a very low failure rate. However, the one issue is that you only use them once and then you discard them. Fundamentally, this screams that there is a cost in the system that shouldn’t be there.
A good analogy is the space industry. It used to take the same approach – use the rocket once and then discard it. Then SpaceX came along and said this is rubbish, we shouldn’t do that. And now, because of that new philosophy of reusing the rockets, SpaceX is now dominating the space industry. It’s a better rocket, of course, but mostly it’s a cheaper rocket.
I think there’s this fallacious perception that, if you make a design sustainable, it should cost more. I think that’s wrong and really shows the limits of present platforms and industry thinking. In my opinion, if it’s good for the environment, it shouldn’t be more expensive – it should cost less.
Q Taking a broad perspective on autoinjectors, how do you rate their historical track record on sustainability?
A Fundamentally this “use once and then discard it” approach is just wrong. Historically, this was never supposed to be the standard. Originally, autoinjectors were designed for emergency use, specifically for soldiers during the first Gulf War. The US military was worried about nerve agents being deployed, so the soldiers on the ground had to be able to inject themselves quickly and reliably in an emergency. In the civilian world, that technology transformed into Meridian Medical Technologies’ (Pfizer) EpiPen (adrenaline), which is another emergency-use scenario.
“I DON’T THINK THAT WHEN THOSE EARLY AUTOINJECTORS WERE DESIGNED, ANYONE BELIEVED THAT THEY WOULD BE PRESCRIBED FOR CHRONIC USE.”
I don’t think that when those early autoinjectors were designed, anyone believed that they would be prescribed for chronic use. For example, in the US last year, there were 6.5 million prescriptions for glucagon-like peptide 1 (GLP-1). If that’s a weekly injection, that’s over 300 million devices for GLP-1 alone.
To put that into perspective, imagine those devices are 10 cm long, so lined up would cover 30,000 km – for comparison, the circumference of the earth is 40,000 km. And the number of prescribed GLP-1s is predicted to grow at a 20% compound annual growth rate. This would mean that, by 2030, there will be a billion single-use devices per year being discarded just for this one therapy.
This isn’t just a cost issue – it’s also an environmental one. These devices are extremely difficult to recycle due to their mix of materials, including non-recyclable hard plastics, metals and glass, some of which qualify as biohazardous waste. If you really want to recycle them, you probably have to take them apart, which is its own challenge with additional associated costs.
So, thinking at the system level, there are probably parts that we can reuse. Some parts, such as the syringe, are inherently single use, but outside the primary packaging we can reuse the mechanism. That’s where there is currently space in the market that I think we should work on.
Q How does Portal balance sustainability with the requirements for device efficacy, safety and usability?
A Again, our approach starts at the system level. The first question is: if we’re building a reusable device, what features are necessary to make it cost-effective? The key is to keep the disposable component as simple as possible, using materials that are easier to recycle. Once that principle is set, all the design and functionality should be concentrated in the reusable part of the device and making it intelligent.
This is where the decade of experience we’ve built up with electromechanical systems from working on our PRIME platform comes into play because, not only was PRIME already developed, all we needed to do was scale it back and make it more cost efficient. We were even able to reuse the same software just by adjusting it so that it’s properly adapted for injection via a needle and syringe – a needle-free system needs a burst of energy to create a jet fast enough to pierce the skin, which you don’t need with a needle.
Taking this approach allowed us to scale back the device by about a factor of five – Nexus is five times smaller and five times cheaper than PRIME. All the technology is still there, and the disposable part is just two pieces of plastic with some clever interlocks that enable the device to manipulate the primary container and perform the injection.
Q Connectivity, which is a key aspect of Portal’s device offering, is also often associated with increased cost and increased environmental impact – do you think that assumption is fair?
A I think it’s true for the current state of affairs where a significant majority of all self-injections are performed with a single-use autoinjector. With a single-use device, connectivity adds more cost and more inconvenience because the patient needs to connect anew to every device. Even with clever technologies, such as Bluetooth Low Energy, that make connecting very easy, it’s still one more step that the patient needs to do.
The cynical view of course is that the industry has been talking about connectivity for a long time but no one’s really figured it out. However, I think that’s missing the point, in that connectivity needs to be introduced in a sustainable way to really stick, and there hasn’t been a truly sustainable reusable device platform released yet. In essence, there hasn’t yet been an opportunity to truly deploy connectivity in the right way.
However, there’s no doubt that, once you have even the most basic data – injection time and confirmation, for example – it would be transformative. Pharmaceutical companies would know how their drugs are being used. Payers would know whether or not the drug is being administered properly. Physicians would be able to stratify their patient population by how compliant they are and identify the ones who need more guidance and assistance to get the most out of their therapies. I think there’s a bright future for connectivity. Ten years from now, an autoinjector should be sustainable and connected – it should be something that’s part of a patient’s life.
Q Of course, sustainability goes beyond just the device – carbon footprint measurements include packaging, shipping, transport and labelling – how do you think manufacturers should be thinking about that?
A In practice, I think this is the first thing that the industry is going to tackle, which is great to see. Can we effectively use bioplastics? Can we use recycled metals? Can we find a recycling programme to process those? There’s already a lot of effort being put in to address these questions. There’s also the question of take-back schemes, which are an effective way to tackle CO2, but those have proven costly because these devices are difficult to reuse or recycle.
“THE AGE-OLD ADAGE IS THAT IF YOU CAN’T MEASURE IT, YOU CAN’T MANAGE IT, WHICH IS WHY OUR DEVICES INHERENTLY INCLUDE A FEEDBACK LOOP, ENABLING THEM TO PROVIDE A VERY PRECISE AND VERY CONSISTENT INJECTION.”
I think connectivity has a role to play here, where it’s used to track the entire life of the device and see how it’s being used and to see its true end of life. How often do people lose their devices? How frequently do they get broken? With connectivity, we can get real data on this and track the entire lifecycle. If, for example, a reusable cassette has a radio-frequency identification (RFID) chip, it could theoretically be read at the recycling facility before it’s processed.
The age-old adage is that if you can’t measure it, you can’t manage it, which is why our devices inherently include a feedback loop, enabling them to provide a very precise and very consistent injection. But there is also a critical feedback loop between the patient and the physician, and I think there is another one with the environment. It’s important to track what our supply chains really look like. Where do all the components of a device come from? Answering these questions is key in moving towards more sustainable industry practices.
Q Sustainability also extends beyond just CO2 – companies need to have clean, non-polluting processes and products that do not damage the environment. How does Portal address non-CO2 environmental responsibilities?
A In practice, we view it all as part of a whole. For example, think about a device that’s used once and then goes to landfill. As an engineer, I need to understand how landfills work and what processes happen there, if I’m going to understand what’s possible from a design perspective. I think that highlights how end-of-life considerations are genuinely challenging.
We regularly hear from patients that they’re sensitive to these concerns. The number one complaint we get is that, while the device is great and the drug is effective, patients have to throw away a whole device every time they self administer, which is a lot of plastic going to landfill. Attempts have been made with take-back schemes, but very little has stuck or been able to be scaled up to a truly effective level.
That’s why we believe that a reusable device – where the main unit is replaced every three to five years instead of after each injection – is the most effective way to significantly reduce landfill waste. This couples with our second principle: the disposable cassette should be very simple and, ideally, should use a form of bioplastic. I don’t think we’re going to use recycled plastics for medical devices – that tends to be frowned on in the industry – but over the last few years there’s been a strong push to look at bioplastics that are more environmentally sustainable, especially as they’re continually becoming more cost effective.
A I mentioned earlier, cost is the key to unlocking sustainable designs. If it isn’t cost effective, it isn’t going to work. I feel that a reusable, needle-based autoinjector is finally reaching the point where, after a few months of use, the device pays for itself, and that’s really exciting (Figure 2).
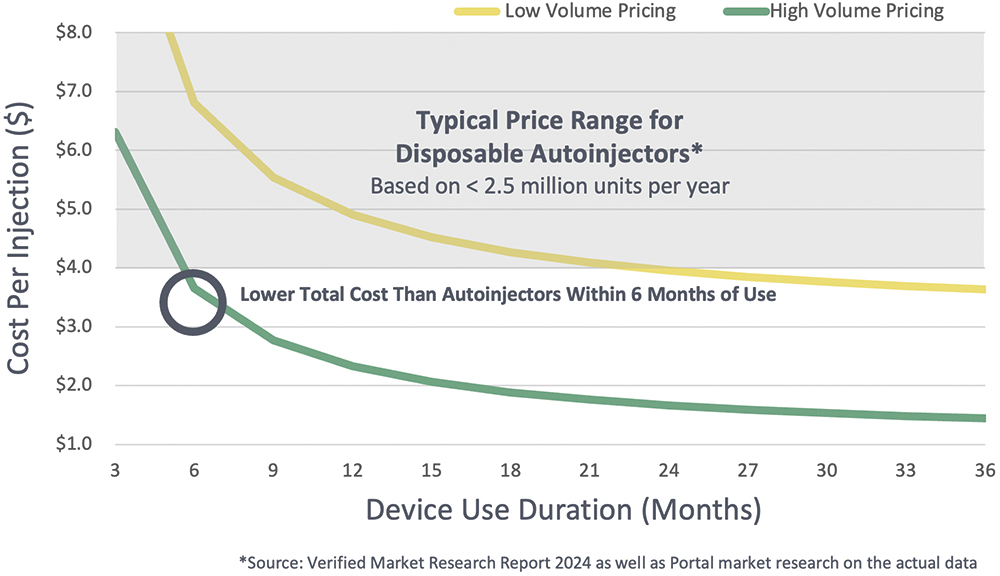
Figure 2: Price per injection over time for a reusable autoinjector.
Q Do you have any final thoughts for our readers?
A I think there’s going to be a significant shift to reusable autoinjectors in the coming years, but they won’t replace disposable devices entirely – I’m not under any illusions that we’re going to be using only reusable devices in the next five to ten years. However, 20 years ago, the market was there for reusable pens and reusable autoinjectors, and patients were using them and getting the job done.
Then there was a shift in the market where everyone thought that making simpler, disposable devices would increase patient compliance, but the evidence suggests that it didn’t pan out that way. I think right now, we’re at a point where the benefits of reusable devices are becoming clear. With the innovation team we have at Portal, I think we’re ideally positioned to seize this opportunity and bring more flexible, more sustainable reusable devices to market and contribute to the next step forward for the pharma industry.

