To Issue 157
Citation: Servol N, “Interview – Unither Pharmaceuticals’ Innovative Approach to Ophthalmic Manufacturing”. ONdrugDelivery, Issue 157 (Mar 2024), pp 25–26.
Q What technologies does Unither offer for ophthalmic products?
A Unither’s industrial engineering offers three main technologies for sterile manufacturing of ophthalmic products: blow-fill-seal (BFS) in single-unit vials, preservative-free multidose (PFMD) filling and common multidose (MD) filling for products with preservatives.
We are always looking for innovative solutions to meet our customers’ requirements and we’re always aware that, ultimately, this equates to meeting patients’ needs. By building industrial synergy between the two main technologies for preservative-free ophthalmic products, BFS and PFMD, we believe we’re truly providing our customers with tailored, sustainable solutions, and patients with products that will enhance their quality of life. This has been the driving force behind the creation and realisation of an innovative PFMD manufacturing line.
“By creating a synergy with unit-dose vials, the PFMD presentation acts as a complement to BFS technology and gives patients continual security and product quality throughout the treatment period.”
Q Can you tell us more about the PFMD line?
A We are proud and delighted with our cutting-edge PFMD line, which is an embodiment of more than 30 years of sterile know-how. It represents uncountable hours of teamwork with external suppliers and customers (Figure 1).
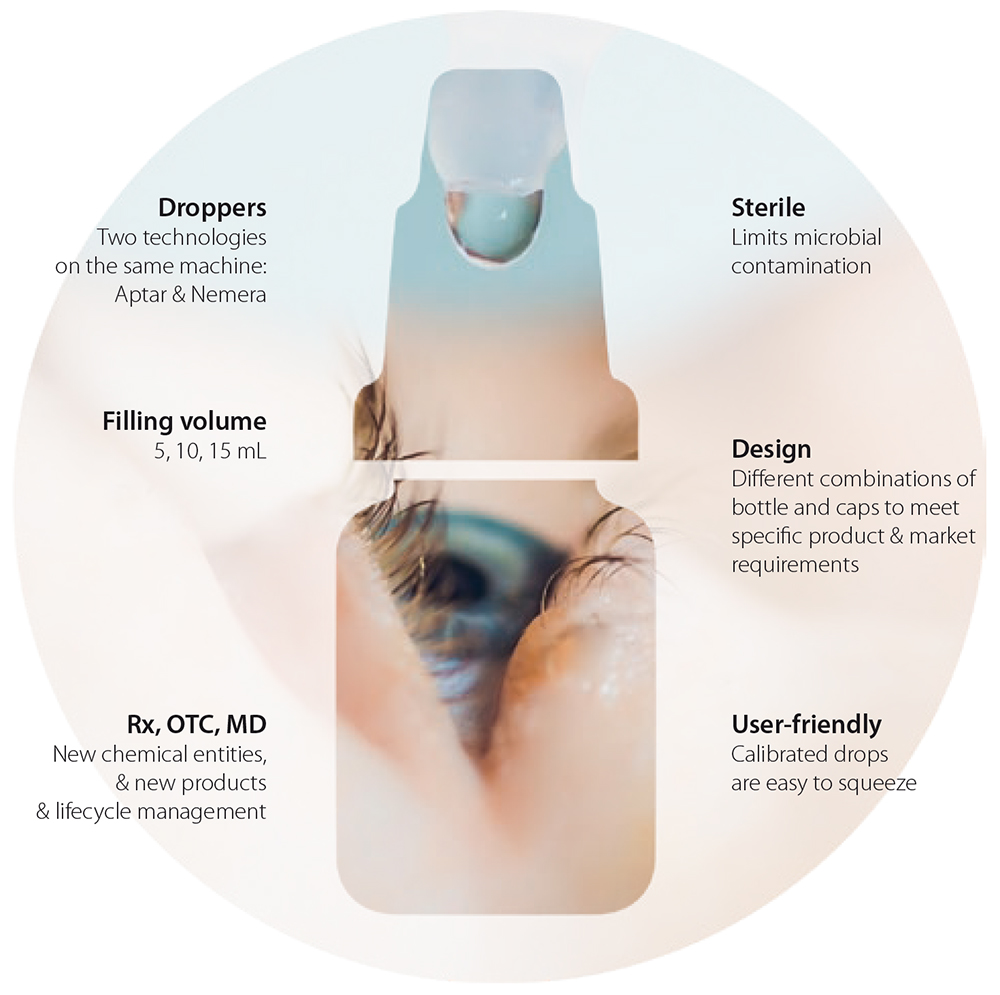
Figure 1: Unither’s innovative PFMD manufacturing line is the embodiment of 30 years of know-how.
“Our R&D site in Bordeaux has made a strategic move and the whole team is working hard to turn our Bordeaux site into an R&D Centre of Excellence for Ophthalmology.”
By creating a synergy with unit-dose vials, the PFMD presentation acts as a complement to BFS technology and gives patients continual security and product quality throughout the treatment period.
The PFMD product is manufactured by aseptic processing. Aseptic manufacturing and the sterile fill-finish process, in which the drug product, container and closure/cap are first subjected to separate sterilisation methods – appropriate to each component and its requirements – and then brought together by the isolator machine.
Q How did you develop this process?
A The PFMD line at Unither is the result of an idea, customer feedback and demands to meet patient needs. As of today, we work with technologies from two major companies in the ophthalmic industry: Aptar Pharma and Nemera. However, our patient-oriented philosophy means that new tailored solutions will be added to our offering in the near future.
Q How do you respond to customers’ needs?
A Every one of our manufacturing plants (in France, the US, Brazil and China) has a dedicated R&D team and pilot workshop for internal development and customer projects. If required, some works can be provided by or executed with the participation of our innovation and development centre in Bordeaux (France), or the back-up manufacturing plant. Our customers benefit from the R&D and international industrial footprint of Unither, from early-stage work to commercial manufacturing.
We answer and meet all relevant international quality and regulatory standards – are approved by the EMA, US FDA, the ANVISA (Brazil), MFDS (Korea), MoH (China) and many others.
We work in an international environment, both for transversal project management and commercial supply.
Q What is next in line for Unither?
A Plenty of exciting projects! Our R&D site in Bordeaux has made a strategic move and the whole team is working hard to turn our Bordeaux site into an R&D Centre of Excellence for Ophthalmology. We keep creating innovative solutions together with our partners. Among many exciting developments, we’d like to mention Curecall (Paris, France), a start-up that specialises in the monitoring of chronic ocular diseases – a user-friendly solution for doctors and patients.
We foresee great challenges in the ophthalmic field and believe that vision science can overcome them by the collaboration and free sharing of ideas within interdisciplinary teams. Our philosophy is to always be open minded.
We will be delighted to discuss these ideas at the ARVO annual meeting and other specialised events.


