Citation: Thompson I, “Large-Volume Patch-Injection via YpsoDose: Simpler for Pharma Companies & Patients. ONdrugDelivery Magazine, Issue 100 (Sep 2019), pp 88-91.
Ian Thompson updates us on the company’s prefilled large-volume patch injector platform, YpsoDose, which simplifies the approach to wearable patch injectors for both pharma companies and patients.
PATCH INJECTORS MEET MARKET DEMAND
The evaluation and selection of a wearable patch injector continues to compete against more frequent dosing based on standard prefilled syringe-based auto-injected therapies. And, for pharma companies to consider and invest in patch injectors they need to be able to access reliable device technology, utilise standard filling processes and, last but not least, fully understand patient and healthcare practitioner (HCP) preferences.
“Whereas the drug in the prefilled syringe is directly connected to the fluid path, within the YpsoDose Needle Unit the fluid path is completed only on injection. The cartridge does not interact with the rest of the YpsoDose injector until the actual time of injection.”
Fulfilling these requirements with the appropriate device technologies will allow the patch injector market to grow significantly over the coming years and become established as a third self-injection device class to complement the well developed markets for pens and autoinjectors.
The number of large-volume injectable drugs, mainly antibody-based, in pharmaceutical development is large and growing. They are in development for the treatment of diseases such as rheumatoid arthritis, psoriasis, IBD/Crohn’s disease, asthma, dermatitis, cardiovascular diseases and migraine. Looking into the future their demand will be further increased by immuno-oncology drugs as maintenance therapies for treated cancers.
General expectations for these drugs is that they will be dosed subcutaneously every two weeks, monthly or even less frequently; that they will be in the range 3-10 mL; and that the injection time will be in the range 3-30 minutes.1 The large injection volume and longer injection time compared with autoinjectors means that the injection system needs to be worn on the skin during administration. For larger injectable volumes, patch injectors require a new drug reservoir, and the prefilled cartridge is the drug container of choice. As a cartridge does not have an integrated fluid path/needle, the patch injector provides the sterile fluid path and injection needle.
LARGE-VOLUME SC INJECTION CHARACTERISTICS

Figure 1: YpsoDose, the prefilled motordriven
patch injector.
Biologics and mAbs have a large therapeutic window and allow the use of a large fixed-dose drug payload compatible with a patch injector. The overall dose and protein concentration may be quite high impacting drug stability and viscosity, drug processing and injection forces.
For example, the protein concentration of blockbuster biologic drugs such as adalimumab and trastuzumab is in the 50-150 mg/mL range and total payloadsfor a single dose may be high as 600 mg or greater. A positive development trend is also the general move from other routes of administration such as IV infusions to subcutaneous administration, in order to reduce the higher proximal and physical administration costs.2
Whatever the type of subcutaneous therapy, there are a number of established therapies, which confirm that the overall injection flow rates for such drugs are in the 0.33-1.00 mL/min range. Examples include: immunoglobulins that are injected at 20-30 mL/h or 1 mL/2-3 min; 3 mL of evolocumab is injected in nine minutes; and 5 mL of trastuzumab containing hyaluronidase is injected at approximately 1 mL/min.
OVERVIEW OF THE YPSODOSE PATCH INJECTOR
Ideally, the less frequently used patch injector should be as easy to use as a disposable two-step autoinjector. The key technical features and benefits incorporated into the prefilled YpsoDose (Figure 1) format are shown in Box 1. They are enabled by YpsoDose’s proprietary electromechanical systems.
Simplifying Drug Filling & Final Assembly
The ability to prefill the drug reservoir and maintain the sterility of the drug reservoir and fluid path during the lifetime of the device is a notable crucial characteristic. YpsoDose achieves this by incorporating a bespoke sterile fluid path enclosed within the Needle Unit. (The Needle Unit can be compared to the staked needle and rigid needle shield of a prefilled syringe.)
Whereas the drug in the prefilled syringe is directly connected to the fluid path, within the YpsoDose Needle Unit the fluid path is completed only on injection. The cartridge, being a well characterised container closure system, does not interact with the rest of the YpsoDose injector until the actual time of injection.
The standardised interface between the 10 mL cartridge and Needle Unit has been designed to allow the cartridge to be filled on conventional filling equipment using ready-to-fill tub formats. Ypsomed is working closely with partners to ensure that standard components and processes are compatible with the YpsoDose device:
• The 10 mL glass cartridge is compatible with standard 13 mm coated vial stoppers and 20 mm coated plungers.
• The pre-crimped cartridges are supplied in a standard three-inch tub format compatible with established filling processes.
• Cartridge characterisation and filling work is ongoing with customers and contract partners.
YPSODOSE USABILITY UPDATE
Current patch injectors are generally HCP or patient filled or assembled; and no prefilled, ready-to-use wearable devices are currently approved for use by patients. Ultimately, to ensure that patch injector therapies are going to be adopted widely for biological therapies, usability is the most important aspect that needs to be successfully tested with patients.
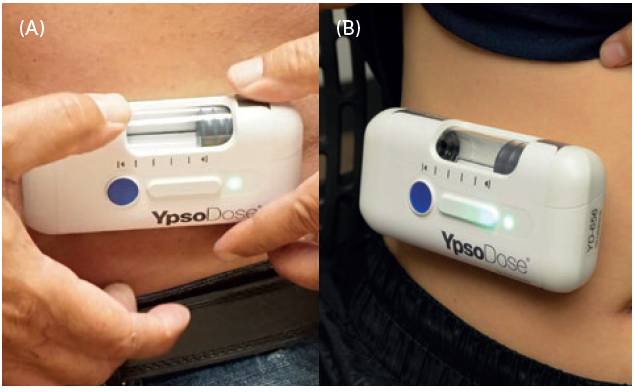
Figure 2: YsoDose being applied (A) and injecting (B).
Continuing human factors work with YpsoDose is proving and optimising the patch system and user interface (Figure 2). The skin sensor system is key to ensure that the injection can only be initiated once YpsoDose is correctly attached to the skin, and to minimise the number of steps required to perform the injection. “Whereas the drug in the prefilled syringe is directly connected to the fluid path, within the YpsoDose Needle Unit the fluid path is completed only on injection. The cartridge does not interact with the rest of the YpsoDose injector until the actual time of injection.”
BOX 1: YPSODOSE KEY TECHNICAL FEATURES AND BENEFITSw
- Prefilled and fully disposable to remove any need to assemble the drug reservoir and device.
- Adheres well to the skin during injection and is easy to remove after injection.
- A capacitive sensing patch which only allows initiation of the injection after the skin sensor has been activated.
- Onboard electronics provide audible and visual signals to clearly communicate with the user before, during and after the injection.
- Automatic insertion of the injection needle at the start and retraction at the end of the injection process. The needle is also retracted if the device is removed from the skin before the end of injection.
- An electromechanical drive accommodates a range of fill volumes and viscosities and provides a programmable and reproducible injection time for each drug.
- The electronics also allow wireless connectivity as required.
All-in-all, YpsoDose’s handling steps are like a two-step autoinjector: remove the cap and inject. For YpsoDose this is simply patch and inject. All other steps are controlled by YpsoDose, which guides the patient when to push the injection button and provide feedback throughout the injection process. At the end of injection the needle is retracted and YpsoDose is ready for disposal or specialist recycling.
In July 2019, YpsoDose 10 mL was tested in the latest formative usability evaluation in the US, to explore the overall handling and safe and effective use in a simulated use scenario, as well to determine the acceptability of the device size and weight. The study included 17 patients from a broad range of disease states, and six HCPs who work with oncology patients.
The results (Table 1) and feedback were positive, and included that:
- All participants completed successful injections following initial training.
- From the HCP perspective, using YpsoDose was seen as a simple and easy way for patients to receive injections or self-inject.
- The user interface is simple and easy to understand, and the orientation of the device was intuitive while placing it on the body.
- Participants were comfortable with YpsoDose’s size and weight, especially with the understanding that it is a singledose, infrequent injection. Participants were positively surprised by how well YpsoDose could be worn on the body to deliver the dose and throughout the study activities.
- Participants viewed YpsoDose favourably in terms of overall design. It was simple to use, communicated how it was to be used, and it was not perceived to be “medical”.
| Question | Average Score | |
| Usability | I felt confident using this device | 4.76 |
| It was easy to keep track of what step I was on with this device | 4.65 | |
| Wearability | It was easy to place the device on my body | 5.00 |
| It was easy to check the device while it was on my body | 4.94 | |
| After use | It was easy to detect when the device had finished the injection | 4.94 |
| It was difficult to remove the device from my skin | 1.94 |
Table 1: YpsoDose patient ratings for Usability, Wearability and After Use. Participants (N=17, adult, adolescent and elderly patients) rated their level of agreement with the statements from 1 (strongly disagree) to 5 (strongly agree).
In summary, the latest 2019 usability evaluation confirms earlier testing performed with previous functional prototypes. The 10 mL YpsoDose fully functional prototypes are now being tested by pharma customers and contract partners. Ypsomed is committed to the successful development and commercialisation of YpsoDose as a new state-of-the-art patch injector.
REFERENCES
- Mathaes R et al, “Subcutaneous Injection Volume of Biopharmaceuticals-Pushing the Boundaries”. J Pharm Sci, Aug 2016, Vol 105(8), pp 2255–2259.
- Tetteh EK, Morris S, “Evaluating the administration costs of biologic drugs: development of a cost algorithm”. Health Econ Rev, Dec 2014, Vol 4(1), p 26.

