Citation: Jeangoudoux I, Phole S, “Launching a Large-Volume Prefillable Glass Syringe System: Reliable Subcutaneous Drug Delivery”. ONdrugDelivery, Issue 178 (Oct 2025), pp 91–94.
Isabelle Jeangoudoux and Sven Pohle introduce SCHOTT Pharma’s new syriQ BioPure® 5.5 mL staked-needle prefillable glass syringe and explain how this solution meets the growing need for large-volume subcutaneous drug delivery.
Subcutaneous (SC) drug delivery is growing as an alternative to intravenous (IV) delivery. It supports the trend to decentralised care, allowing injection therapy to take place outside of the hospital and potentially in the home. Using prefilled syringes (PFSs) as an alternative to vials for SC formulations reduces manual preparation steps and minimises medication errors during administration, further supporting at-home injections and providing a patient-centric solution with greater convenience and comfort. Handheld autoinjector devices designed for use with PFSs have been instrumental in shifting the point of care from hospital to home and have become a preferred solution for safe and effective self-administration.1
Where SC dosing volumes had previously been limited to 1 mL and later 2.25 mL, new co-formulation and enabler technologies have been developed that allow the injection of larger volumes, exceeding this range significantly. Since high-dose drug applications are usually associated with higher viscosities of the formulations, new requirements are now being placed on large-volume drug delivery solutions.
MEETING THE NEEDS OF A GROWING MARKET
The advances in drug formulation development and new therapy concepts are reflected in a growing pipeline of large-volume SC drugs. A recent review identified 182 approved or clinical-stage large-volume drugs either currently using the SC route or with high potential to use this administration route. Of these, 31 were approved large-volume SC agents and 83 were clinical-stage candidates, demonstrating the potential for significant growth in this area.2 Another 68 IV formulations were identified by the study authors as potential candidates for transition from an IV to an SC route. The review found that of these 182 drugs, 81 had a volume range between >2–5 mL, while the remainder had even larger volume ranges. These trends indicate a significant need for large-volume container solutions that can handle volumes exceeding 2 mL at a wide range of viscosities and that are compatible with SC injection devices, such as autoinjectors.
“A PROVEN CONTAINER PLATFORM CAN FACILITATE A SHORTER TIME TO CLINICAL TRIALS.”
Time-to-market is critical for biopharma companies, and because clinical testing is a lengthy process, companies want to reach this step as quickly as possible. A proven container platform can facilitate a shorter time to clinical trials. This makes available multiple configurations of the primary packaging to best meet specific customer needs and provide a prequalified route to easy sampling, regulatory filing and commercial supply. Alongside this, there is a need for dependable production capacity as well as technical and regulatory support for biopharma companies and CMOs entering the growing market of large-volume SC drug delivery.
The latest solution from SCHOTT Pharma is the syriQ BioPure® 5.5 mL staked-needle glass PFS, designed to meet the growing need for large-volume SC drug delivery (Figure 1). SCHOTT Pharma brings all the knowledge gained from the established 1 mL long and 2.25 mL PFS glass platforms for biologics to the development of the 5.5 mL format.
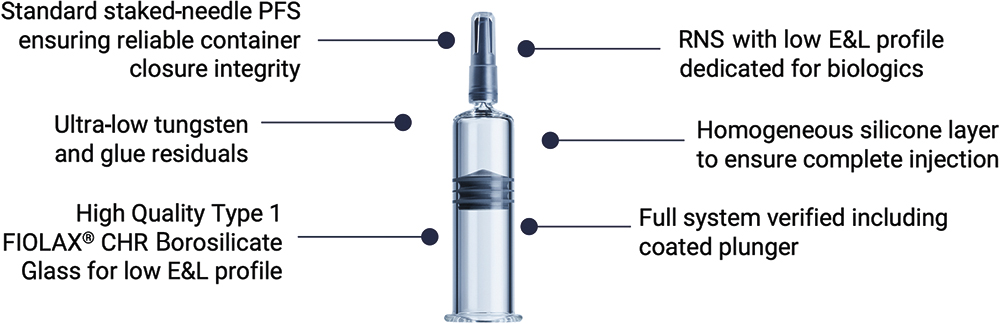
Figure 1: Key features of the syriQ BioPure® 5.5 mL prefillable syringe, a new, large-volume drug delivery solution in the syriQ BioPure® platform that builds on the proven success of the 1.0 mL and 2.25 mL syringes.
“IN THE HOLISTIC DEVELOPMENT OF THE NEW 5.5 ML STAKED-NEEDLE GLASS PFS, SCHOTT PHARMA CONSIDERED ALL THESE FACTORS AND COLLABORATED WITH ITS PARTNERS TO IDENTIFY THE BEST SOLUTION.”
When developing a PFS, the needs of all stakeholders should be taken into account. Firstly, drug compatibility and safety must be ensured. Secondly, the patient and user needs are key, including comfort and ease of use, as well as considering how to build confidence for the user. Processability throughout fill-and-finish and final assembly stages must be evaluated, as well as business aspects, such as speed of implementation and lowering development risks. For a PFS designed for use in an autoinjector, collaboration between the container and device developers is crucial. In the holistic development of the new 5.5 mL staked-needle glass PFS, SCHOTT Pharma considered all these factors and collaborated with its partners to identify the best solution.
PURITY FOR SENSITIVE DRUG FORMULATIONS
Minimising interaction between the drug and the container is of primary importance and a key pharmaceutical requirement. A low extractables and leachables (E&L) profile of all components of the primary packaging is crucial for many drugs used in SC drug delivery because these biopharmaceutical formulations may be sensitive to extractables from the glass or the elastomeric closures. Therefore, first class components and materials have been chosen for the design of syriQ BioPure® 5.5 mL.
The glass barrel uses Type I FIOLAX® CHR Borosilicate Glass that has been designed for controlled hydrolytic resistance of the inner surface of the container. In addition, SCHOTT Pharma’s converting processes for high-value prefillable glass syringes enable ultra-low tungsten levels. This is essential because tungsten oxide is a critical residue for some biological drugs; even amounts in the nanogram range can cause aggregation, denaturation or precipitation of proteins, such as monoclonal antibodies, which might increase the risk of immunogenicity.3,4 In principle, tungsten residues may originate from the forming pins during hot forming of the syringe cone. Through the development of an alternative pin alloy, SCHOTT Pharma has established a high-quality forming process for the whole syriQ BioPure® platform so that an ultra-low tungsten specification can be ensured.
For the elastomeric components, state-of-the-art materials were selected, such as Aptar’s (IL, US) Rigid Needle Shield (RNS) 4900GS. This uses a modern styrene butadiene rubber material with a type I classification according to EP 3.2.9 and US Pharmacopoeia Chapter <381> and a fit-for-biologics positioning. Datwyler’s latest plunger development for large-volume PFSs was also selected, which is a NeoFlex™ plunger coated with a protective fluoropolymer layer, typical for use with biologics to minimise interactions with sensitive drugs.
FUNCTIONALITY IS KEY FOR PATIENT-CENTRIC DRUG DELIVERY SYSTEMS
“THE TEST GIVES CONFIDENCE THAT THE NEW 5.5 ML SYRINGE AND THE LARGE-VOLUME NEOFLEX™ PLUNGER FROM DATWYLER MEET THE REQUIREMENTS FOR GLIDING FORCE THROUGHOUT ITS SHELF LIFE.”
To address user needs in terms of confidence and ease of use, the syriQ BioPure® 5.5 mL syringe system was carefully designed for reliable functionality over its entire shelf life. One essential requirement is, of course, that the patient receives the full injection from the autoinjector. Therefore, the gliding performance of the plunger in the spray-coated syringe barrel must be reliable and consistent over its shelf life. Exemplary results for water-filled syringes stored for up to six months at 5°C and 25°C are shown in Figure 2. Average and maximum gliding force did not change significantly during storage at either temperature or compared with initial performance. The test gives confidence that the new 5.5 mL syringe and the large-volume NeoFlex™ plunger from Datwyler meet the requirements for gliding force throughout its shelf life and function as a reliable container system in the autoinjector.
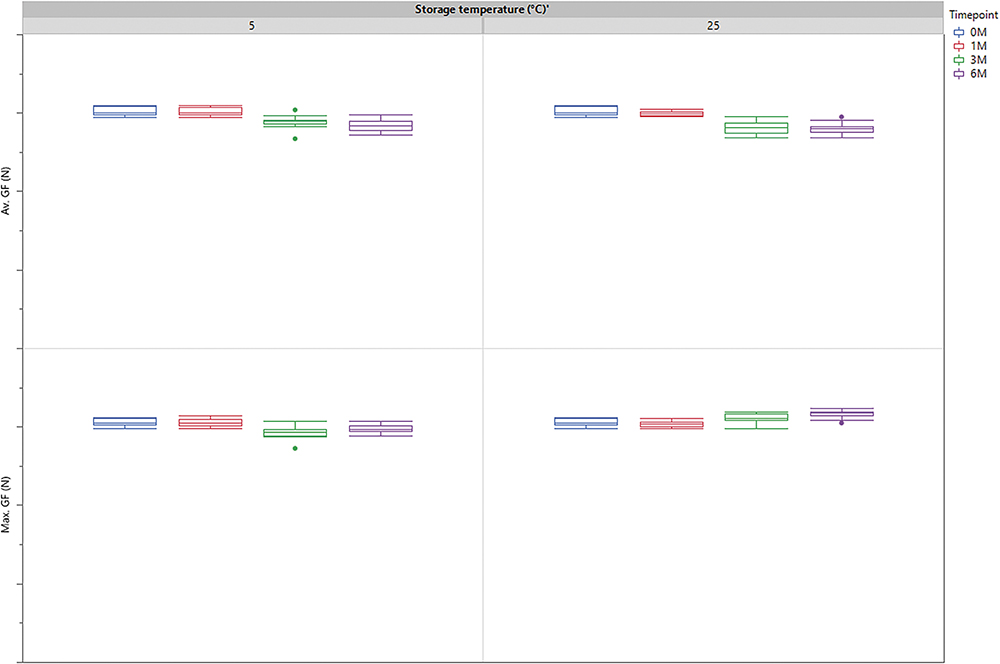
Figure 2: The gliding force (GF) for water-filled 5.5 mL syringes was tested on a universal test machine directly after filling (0 m) and after 1, 3 and 6 months of storage at 5°C and 25°C. The syringes were stoppered with a Datwyler NeoFlex™ plunger.
For the application of more viscous drugs, the needle’s inner diameter plays a crucial role, as it impacts the hydrodynamic extrusion force significantly. Therefore, the syriQ BioPure® 5.5 mL features a high quality, 27G ½-inch special thin wall needle. To reduce the extrusion force, this needle has an increased inner diameter compared with a standard 27G thin wall type.
Another important factor for the user experience is the removal of the autoinjector cap. In addition to specific design requirements for the device, the interaction of the RNS with the syringe cone is also relevant here – a low RNS pull-off force supports a low cap removal force with the autoinjector. Verification studies confirmed that the 4900GS RNS meets the quality requirements.
Furthermore, dosing accuracy is an important user requirement. The syringe system contributes to this with a minimal residual volume smaller than 20 μL, which is <0.4% of the total syringe volume. This low residual volume has been achieved by an ideal match of barrel shoulder dimensions with Datwyler’s large-volume NeoFlex™ plunger. The plunger design incorporates a dome and optimised dimensions allowing it to fit perfectly into the syringe barrel design.
CO-DEVELOPING WITH AN AUTOINJECTOR
Autoinjector devices are commonly used for at-home injection. The interplay of device and container is crucial to ensure safe and reliable drug administration during self-medication. Therefore, the interfaces between autoinjector and PFS must be clearly matched and understood as a system that, as a whole, ensures essential performance requirements are met.
SCHOTT Pharma and Ypsomed collaborated to co-develop the new syriQ BioPure® 5.5 mL PFS and the YpsoMate® 5.5 autoinjector. As part of this co-development process, the critical syringe dimensions for autoinjector use and optimal performance criteria were determined and included in a joint interface specification. Precision is a key requirement for the container-device interaction, as it ensures reliable processability in the syringe-to-device assembly, as well as functionality and ease of use. In addition to the interface specification, compatibility tests were performed at Ypsomed during the development process, to ensure that the two parts would function together smoothly.
SIMPLIFYING THE FILL-AND-FINISH PROCESSES
Thinking in systems means considering the whole ecosystem of a PFS. Therefore, it is important to assess the impact of the new large-volume syringe on the fill-and-finish landscape. Firstly, the design of syriQ BioPure® 5.5 mL allows the use of SCHOTT Pharma’s standardised secondary packaging, the SCHOTT iQ® platform. All three syringe formats of the biologics PFS glass platform – 1.0 mL long, 2.25 mL and now 5.5 mL – are packaged in a standardised 3” tub for simple incorporation into existing fill-and-finish systems. The new 5.5 mL design fits 42 syringes in the standardised tub (Figure 3). Secondly, throughout industrialisation of syriQ BioPure® 5.5 mL, various partners in the fill-and-finish sector have successfully conducted technical test runs and begun introducing the new large-volume syringe format.
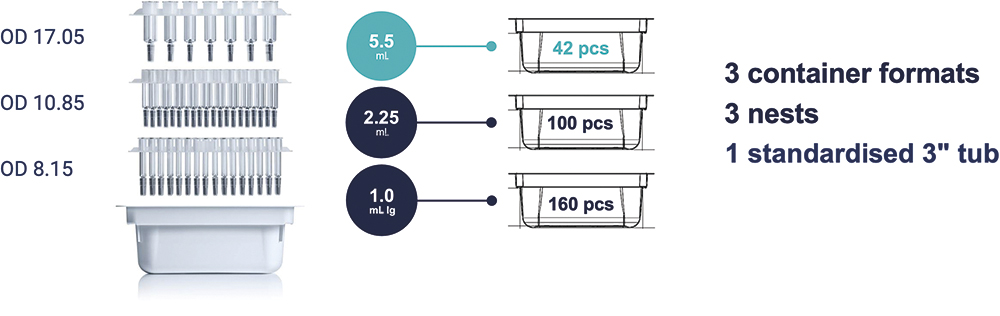
Figure 3: The syriQ BioPure® platform uses a standardised 3″ tub for all three volumes of pre-sterilised, nested prefillable syringes, designed to facilitate automated fill-finish. The tub is sealed with a Tyvek® sheet and packaged in a double-bag configuration. OD: outer diameter of the glass barrel.
CONCLUSION
SCHOTT Pharma’s syriQ BioPure® 5.5 mL is the first large-volume staked-needle glass PFS designed for SC drug administration of biologics. The new PFS, co-developed to be compatible with the YpsoMate® 5.5 autoinjector, enables a broader design space for formulation development and improved patient-centric drug delivery for large-volume SC therapies. These advances reduce the treatment burden for patients by allowing fewer injections at home for greater comfort and convenience. As part of a proven PFS platform, this new large-volume syringe offers reliable performance in terms of drug compatibility, safety, functionality and processability in final assembly and fill-and-finish. The platform approach allows a fast time to market and comes with reduced risks for combination product development. SCHOTT Pharma provides expertise in large-volume container fill-and-finish, along with regulatory support, so that biopharma companies and CMOs can quickly come up to speed with this new format and achieve fast implementation with low risk. This new PFS provides a solution for the growing pipeline of large-volume SC drugs, ultimately enabling solutions that reduce the treatment burden for patients.
SCHOTT Pharma is currently in the industrialisation phase. Not-for-human-use samples of the 5.5 mL PFS are available for testing and feasibility studies. The commercial launch of for-human-use product is expected by the end of 2025.
REFERENCES
- Schneider A et al, “Autoinjectors for large-volume subcutaneous drug delivery: a review of current research and future directions”. Expert Opin Drug Deliv, 2023, Vol 20(6), pp 815–830.
- Green P et al, “Navigating large-volume subcutaneous injections of biopharmaceuticals: a systematic review of clinical pipelines and approved products”. MAbs, 2024, Vol 16(1).
- Bee et al, “Precipitation of a Monoclonal Antibody by Soluble Tungsten”. J Pharm Sci, 2009, Vol 98(9), pp 3290–3301.
- Seidl A et al, “Tungsten-Induced Denaturation and Aggregation of Epoetin Alfa During Primary Packaging as a Cause of Immunogenicity”. Pharm Res, 2012, Vol 29(6), pp 1454–1467.

