To Issue 157
Citation: Davidson Z, “Listening to the Patient’s Voice to Improve Eye Care”. ONdrugDelivery, Issue 157 (Mar 2024), pp 10–14.
Zoë Davidson discusses the benefits of collaborating with patients to improve eye care management.
Growth in the global ophthalmic eyedropper market is expected to be driven by the increasing prevalence of eye diseases and disorders such as glaucoma, dry eye disease and age-related macular degeneration. Moreover, ageing populations are further propelling market growth due to a higher incidence of age-related eye conditions.1
“Drug delivery device designers and manufacturers must strive not only to understand but to provide solutions for patients encountering administration challenges and to improve at-home eye care management.”
Glaucoma, which is the leading cause of irreversible blindness worldwide, refers to a group of optic neuropathies characterised by the progressive degeneration of retinal ganglion cells. Open-angle glaucoma is the most common form of glaucoma, carrying a chronic prognosis and making up 75–95% of primary cases.2 Angle-closure glaucoma can be either acute or chronic, but typically has a faster progression than open-angle glaucoma and, therefore, requires more drastic interventions. These two main forms often require the use of eye drops to reduce intraocular pressure and prevent further damage. With the ageing population, the demand for glaucoma medications administered via eyedroppers is also expected to increase, thereby driving market growth. For example, people are six times more likely to develop glaucoma after the age of 60.3
The dry eye segment dominated the ophthalmic market in terms of revenue in 2023, owing to its rising prevalence.1 The definition of a dry eye, according to the Tear Film and Ocular Surface Society Dry Eye Workshop II, is: “Dry Eye is a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiologic roles”.4
One increasingly common extrinsic risk factor for dry eye is digital screen use (e.g. computer, laptop, tablet and smartphone use), which is thought to contribute to its development by affecting blinking dynamics.5 Other factors contributing to dry eye include ageing, air pollution and hormonal changes. Eye drops or artificial tears are the primary treatment options for alleviating symptoms by lubricating the eyes and providing moisture.1
Given the growing ophthalmic eyedroppers market – owing largely to the increase in the ageing population – drug delivery device designers and manufacturers must strive not only to understand but to provide solutions for patients encountering administration challenges and improve at-home eye care management. One way of gaining insight into the patient journey is via testimonials and reviews of real patients using existing products on the market.
Figure 1: Systane™ hydration PF multidose lubricant eye drops for the treatment of dry eye launched in the US market in 2021 with Novelia® (image courtesy of Alcon).
ACKOWLEDGING PATIENT PAIN POINTS TO IMPROVE EYE CARE MANAGEMENT
As patients become increasingly informed and discerning, they come to rely heavily on the experiences and opinions of others to make well-informed choices. A key advantage the strong position Nemera’s Novelia® holds as a leading multidose eyedropper for preservative-free (PF) formulations is having access to a cornucopia of verified patient reviews on e-commerce websites and social media platforms. This is especially true for over-the counter products for the treatment of dry eye.
Products on the market using the Novelia® device consistently rate highly among patients. For example, Systane™ complete PF multidose dry eye drops have maintained an average star rating of 4.5/5, across over 10,000 verified customer reviews since its market launch in 2022, making it the number one product in the moisturising eye drops category on Amazon US (Figure 1).
Online reviews by patients are an invaluable asset for Nemera, providing insights into real patient experiences. Even less-than-positive aspects offer valuable feedback for improvement that Nemera embraces as an opportunity to identify weaknesses and make meaningful improvements to its products and services.
“Patients and healthcare professionals alike desire a bottle that allows patients to control the number of drops delivered, consistently delivering a single drop with each actuation.”
Preservative-Free
The majority of eye drops today contain preservatives to maintain the sterility of eye drop formulations. The most commonly used preservative is benzalkonium chloride, which has been known to damage the cornea with long-term use.
Preservatives can also cause allergies or ocular irritation, and some can even cause a toxic response.6 Any such reactions are issues for patients who rely on the long-term use of eye drops for chronic conditions.
Control
Patients and healthcare professionals alike desire a bottle that allows patients to control the number of drops by consistently delivering a single drop with each actuation. A study of glaucoma patients found that 90% used an erroneous technique, with many patients missing the eye entirely.7
Novelia’s PureFlow™ technology not only serves as a venting system but also controls medication flow (Figure 2). Nemera has adapted the flow control within Novelia® to avoid multiple drop delivery into the eye and ensure that only one calibrated drop is dispensed at a time. Specifically, Nemera offers three different PureFlow™ versions, each tailored to formulations of differing viscosities, from highly fluid to highly viscous. In addition, five different valve sizes are available, each one delivering a different calibrated drop size. This allows Nemera’s team to customise the drop size depending on specific product requirements. This improved control leads to increased patient confidence (of accurate dosing) and reduced frustration and medication waste.
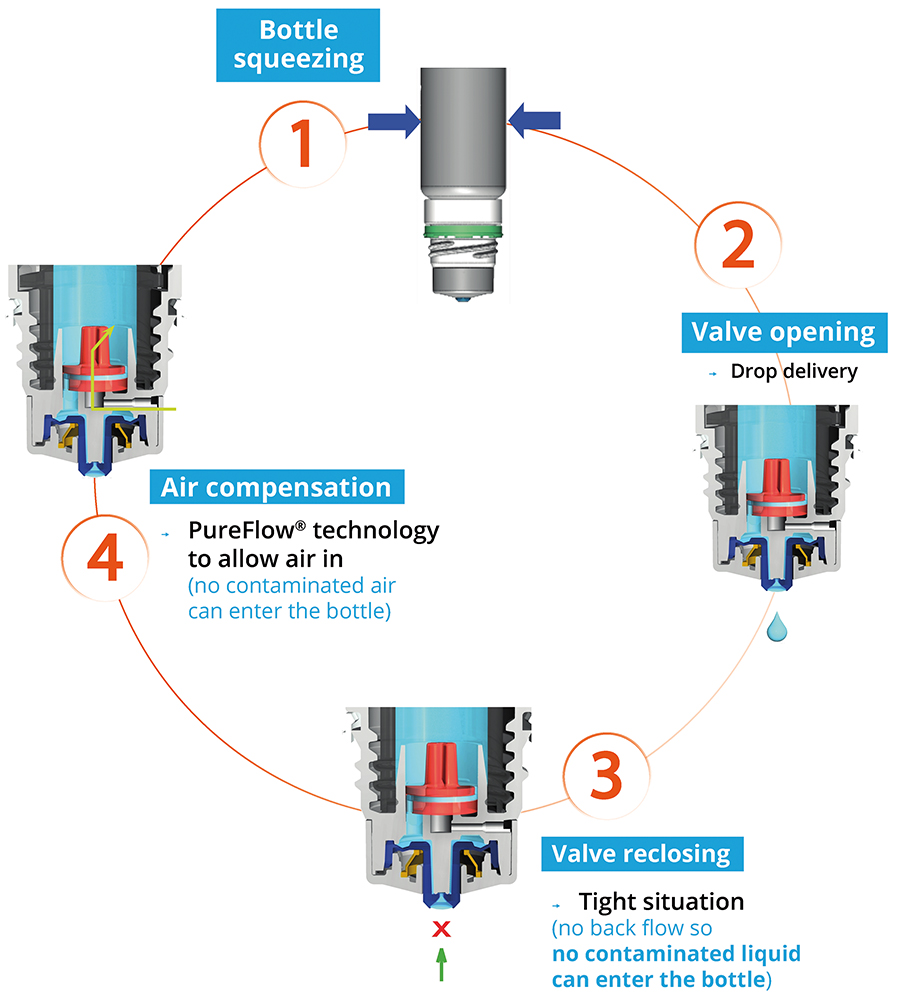
Figure 2: The Novelia® system uses a non-return valve that removes the need to filter the liquid.
Ease of Use
Patients, even those with dexterity issues and tremors or shaking, must be able to manipulate the delivery system effectively and administer a single drop. Contributing factors to the preference for Novelia® by 76% of patients included the intuitiveness of the screw-on cap and the associated reassurance and squeeze force required towards the product’s end of life. Novelia® required only 6% more pressure to squeeze the bottle at the end of the treatment compared with at the beginning, whereas for other PF multidose bottles, the increase in required pressure was 35%.8
For example, one verified Amazon US customer, in her review of Systane™ hydration PF, said: “So delighted with this packaging. Have used a competitor’s product and I had to use 2 hands to squeeze the bottle. (I am 83 with very little grip strength.) This bottle is much softer, and I am able to use 1 hand which easily leaves my other hand free to ‘open’ my lower lid and instil the drops in the appropriate way. I don’t care for the individual PF drop containers as they are more expensive when using throughout the day and I found those containers were also difficult to squeeze.”
Novelia’s® patented blue tip is also a favourite feature of the device. It helps patients target the eye before drop administration and anticipate the angle of the drop on to the ocular surface. One verified customer on Feefo UK (Butterflies Eyecare), in her review of Evolve SOOTHE & RENEW eye drops 10 mL, said: “The blue dot on the dispenser helps line up the bottle with the eye. Because I can see how far away the bottle is from my eye, I am less threatened by it than a pointed dispenser. Thus, I can relax more when applying drops to my eyes (whether I do it or someone else does). This makes the application process a lot easier.”
Figure 3: A full range of LDPE bottles is available in 5, 7.5, 11 and 15 mL, as well as 11 mL PP.
Transparency
Patients need more visibility into their medication supply, so that they can replenish as needed and do not find themselves without. A full range of bottles is available in terms of size, material and sterilisation type (5, 7.5, 11 and 15 mL). All sizes are available in low-density polyethylene (LDPE) either in white or natural (transparent), allowing patients to know when their medication is running low. Nemera has also developed a polypropylene (PP) 11 mL bottle for specific formulation compatibility (Figure 3). Novelia® bottles have been validated using both gamma and ethylene oxide sterilisation.
Portability
A patient must be able to easily transport their medication; for dry-eye syndrome patients this is likely to be daily, while for glaucoma patients this is likely only for overnight/travel. The Novelia® device features a screw-on cap that fits tightly on to the device nozzle, which is optimal in terms of portability. Other marketed devices that use a snap-on cap have been found by patients to be less robust, with several instances of leaking during transport, whether in a handbag or pocket. As such, Novelia® outperformed another marketed device in terms of cap opening, hermetic sealing and nomadic use, with a mean score of 4.5 out of 5 for the three features.9 One verified Amazon customer said: “Love the portability. It’s nice not needing to carry around small individual containers for each use in your pocket and around the house. I do like that this one (as) you can twist the cap on and off (so it) feels more secured.”
Sustainability
Patients also report concerns regarding unit doses. These include cost (as more packaging and eye drop solution per dose is required), waste and convenience, as it is easier to store a multiuse bottle in a preferred location than to ensure that the patient has the correct number of unit dose pipettes with them every day.10
“Patients need more visibility into their medication supply, so that they can replenish as needed and do not find themselves without.”
Handling difficulties have been noted with unit doses and their use by older patients, and inappropriate finger manipulation could be associated with an increased risk of contamination.11 Due in part to the rapidly ageing population, the number of people with glaucoma worldwide is expected to increase to over 111 million by 2040.12
In an analysis conducted by Nemera, comparing Novelia® multidose eyedropper with unit dose packaging for a glaucoma-type regimen (one drop per eye twice per day) over one month, there was eight times less plastic used, 25 times less drug waste and nine times less energy needed for transportation for Novelia®, compared with a unit dose.13
In 2021, Nemera decided to subscribe to the Science-Based Target initiative (SBTi) to define and develop best practices for carbon reduction. One of the first objectives is to reduce the company’s Scope 1 and 2 emissions by 90% by 2030 from a 2019 base year. In addition, since February 1, 2024, the manufacturing facility in La Verpillière, France, where Novelia® is manufactured has been certified ISCC PLUS. This certification scheme for bio-based, renewable and circular raw materials reinforces Nemera’s implementation of sustainability goals (Figure 4).
Figure 4: Reducing carbon emissions.
SUPPORTING CUSTOMERS TO SUPPORT PATIENT NEEDS WORLDWIDE
Nemera offers a range of laboratory services for Novelia®, including testing of customers’ bulk formulation. Testing comprises usage simulation over two weeks, drop size analysis (variable depending on valve diameter) and flow control and squeeze force testing (beginning and near end of life). Analysis of the results allows Nemera to determine the best Novelia® configuration for a particular customer formulation. Nemera can recommend the most suitable PureFlow™ control, bottle type and valve size to achieve the desired drop calibration.
Nemera’s regulatory team is on hand to support customers with their submission filings, providing guidance on supportive documents for registration. Nemera can also assist customers in finding the right ready-to-go dossier available for private labelling of certain molecules with the Novelia® delivery system. Nemera has a substantial list of partners, formulation licensors and fillers, all working in collaboration to bring to customers a finished drug-device combination product with Novelia®.
While user-friendly containers are important, educational resources that teach patients how to apply their eye drops correctly have been found to mitigate issues with unintentional non-compliance.14 For example, Nemera can support customers with product market launch, in educating sales teams and healthcare professionals on the delivery device, dedicated training and materials to assist in promotional material creation. Customisable patient guidance videos are also available in several languages to increase patient compliance around the world.
Currently, Novelia® has approximately 300 references on the market for prescription and over-the-counter products in over 55 countries across Europe, Latin America, North America, Oceania, the Middle East and Asia Pacific.
To serve customers in supporting patient needs, Nemera has extended its manufacturing capabilities in the US, and in so doing has doubled its capacity to produce Novelia® PF multidose eyedroppers (Figure 5).

Figure 5: Nemera has doubled its Novelia® production capacity by creating two additional assembly lines in their US site, (Buffalo Grove, IL).
REFERENCES
- “Ophthalmic Eye Droppers: Market Analysis Segment Forecast From 2018 To 2023.” Grand View Research, accessed Feb 2024.
- “Glaucoma: Competitive Landscape to 2026”. GlobalData, accessed Feb 2024.
- Allison K, Patel D, Alabi O, “Epidemiology of Glaucoma: The Past, Present, and Predictions for the Future”. Cureus, 2020, Vol 12(11), article: e11686.
- Golden MI, Meyer JJ, Patel BC, “Dry Eye Syndrome” in “StatPearls”, StatPearls Publishing, 2023.
- Al-Mohtaseb Z et al, “The Relationship Between Dry Eye Disease and Digital Screen Use.” Clin Ophthalmol, 2021, Vol 15, pp 3811–3820.
- “Report of the International Dry Eye Workshop”. Ocul Surf, 2007, Vol 5(2), pp 65–204.
- Tatham AJ et al, “Eye drop instillation technique in patients with glaucoma”. Eye (Lond), 2013, Vol 27(11), pp 1293–1998.
- User study performed for Nemera by GfK to understand the Novelia® market opportunities versus competitors (Aptar OSD system)”. GfK report, 2015.
- “User study performed for Nemera by QUALIQUANTICI, Novelia® and a marketed pump product testing”. Marketing Espace report, Lyon, 2018.
- Erb C, “Glaucoma and Dry Eye”, 2020, 2nd Ed, Berlin: UNI-MED Science.
- Campolo A, Crary M, Shannon P,“A Review of the Containers Available for Multi-Dose Preservative-Free Eye Drops”. Biomed J Sci & Tech Res, 2022, Vol 45(1), pp 36035–36044.
- Tham Y et al, “Global Prevalence of Glaucoma and Projections of Glaucoma Burden Through 2040: A systematic review and meta-analysis”. Ophthalmology, 2014, Vol 121(11), pp 2081–2090.
- “Comparison analysis conducted by Nemera comparing Novelia® multidose eyedropper for preservative-free formulations with unit dose packaging,” Internal Research, Nemera, 2020.
- Davis SA et al, “A randomized controlled trial of an online educational video intervention to improve glaucoma eye drop technique”. Patient Educ Couns, 2019, Vol 102(5), pp 937–943.


