Citation: Johnson R, “Nasal Drug Delivery: Overcoming Challenges with a Robust Platform Strategy”. ONdrugDelivery, Issue 154 (Nov 2023), pp 78–81.
Richard Johnson explores the changing nasal delivery landscape, highlighting challenges in nasal drug formulation and potential methods to overcome them.
“Patient-centricity is a priority in the industry, aiming to increase patient access, compliance and adherence. The use of nasal delivery devices is helping to achieve this goal, offering a delivery method that is quick, easy and non-invasive.”
Delivering drug products via the nasal cavity is not a new practice, with nasally administered treatments, such as antihistamines and cold relief medications, readily available over the counter. Now, innovation in the pharmaceutical sector is leading to a shift in exploring the use of nasal devices to deliver a wide range of therapeutics, ranging from traditional small molecule APIs to larger, more complex molecules, such as peptides and vaccines. Moreover, there is a particular interest in delivering molecules both systemically and directly to the brain.
These sensitive drugs often rely on parenteral administration to avoid the harsh conditions of the gastrointestinal (GI) tract. By delivering these drugs via the nasal route, it is possible to avoid the GI tract and first-pass metabolism, as well as making them easier to self-administer. In light of this, the global nasal delivery market is experiencing rapid growth, with its value predicted to increase from US$72.89 billion (£60.05 billion) in 2022 at a compound annual growth rate of 7.45% until 2030.
THE BENEFITS OF NASAL DELIVERY
The nasal cavity enables drug products to be absorbed quickly across the nasal mucosa and then distributed systemically. By avoiding the GI tract, this immediate absorption into the bloodstream ensures a rapid onset of therapeutic effects. Administering drugs into the bloodstream via the nose can also overcome the issues of first-pass metabolism by the liver, an issue that is difficult to overcome with oral dosage forms. Additionally, intranasal delivery offers direct access to the brain, bypassing the blood-brain barrier.
Patient-centricity is an industry priority, aiming to increase patient access, compliance and adherence. Nasal delivery devices are helping to achieve this goal, offering a delivery method that is quick, easy and non-invasive. In contrast with many parenteral delivery methods, nasal delivery devices can also be self-administered, circumventing the need for healthcare practitioner involvement and reducing the burden on healthcare systems.
However, a number of challenges can hinder the development of drug products for nasal delivery. As the industry explores administration systems for the nasal delivery of more sensitive materials, such as vaccines and peptide-based products, innovative solutions are required to successfully formulate and administer them while maintaining their stability and efficacy.
“As drug molecules become more complex, challenges such as solubility and stability are more likely to arise, and alternative solutions to overcome these limitations may be required.”
OVERCOMING NASAL FORMULATION CHALLENGES
Traditionally, nasal products are liquid-based, with the drug product solubilised in water to form droplets when sprayed from the device into the nose. When administered, the drug product is then deposited in the nasal cavity, where it can act on the nasal epithelial surface. From there, the drug product has the potential to be absorbed across the nasal mucosa and transported systemically through the bloodstream, and reach the target to exact a pharmacological effect. Alternatively, the drug product can be administered into the nose as a dry powder, dissolving once the powder particles impact the moist surfaces of the nasal epithelium to release the drug for either local or systemic action.
The target product profile (TPP) outlines the desired characteristics of a drug product. If created early in the development process with a thorough understanding of the properties of the API, the TPP can help to guide development, highlighting which characteristics need to be modified for the drug product to meet target standards. These present some challenges that must be overcome to successfully formulate a drug product for nasal delivery, including:
- Solubility: Poor solubility impacts drug distribution and bioavailability, as well as the choice of device (liquid vs dry powder).
- Stability: Unstable products may lose their activity, with stability often reduced when solvated, necessitating the dry powder approach for delivery.
- Bioavailability: Insufficient bioavailability hinders the delivery of the drug around the body and can significantly impact the therapeutic effect of the drug.
These challenges are commonly interlinked, with issues such as solubility and stability favouring a dry powder formulation that offers the best developmental approach compared with a liquid-based device. As such, some of these factors can be overcome by enhanced formulation approaches. Excipients are added routinely to the final formulation to improve the solubility, stability and bioavailability of the API. For example, a gelling agent can be added to the formulation to increase the residence time of the API on the surface of the nasal cavity, allowing for absorption over a longer timeframe. Other excipients include agents that can enhance membrane permeation by opening up the tight junctions in the nasal epithelium.
To achieve optimal results, the addition of excipients should be coupled with other strategies to infer desired characteristics and form the final formulated product. For example, with dry powder device formulations, the engineering of particle size plays a critical role in ensuring optimal deposition of the drug in the appropriate areas of the nasal cavity while preventing the drug from entering the lungs.
DRY POWDER DELIVERY SYSTEMS OFFER AN ALTERNATIVE SOLUTION
As drug molecules become more complex, challenges such as solubility and stability are more likely to arise, and alternative solutions to overcome these limitations may be required. When the drug product is dissolved in an aqueous carrier, its stability can be reduced in comparison with a dry powder form. These stability problems can result in degradation of the drug product, significantly limiting its shelf life. Therefore, dry powder nasal formulations are increasingly being investigated as solutions for more complex drug products.
Dry powder nasal devices are designed to deliver dry powder particles directly into the nasal cavity by either actively firing the powder into the nose or the recipient inhaling the powder from the device, actively inhaling through the nostril. As the particles exit the device and make contact with the nasal mucosa, particle dissolution occurs, and the drug is absorbed onto the nasal epithelial surface for local action or transport across the membrane and distribution throughout the body. Additionally, the recent emergence of commercially available nasal devices that can deliver their drug product as a dry powder also enables temperature-sensitive products to be transported and administered without the need for cold chain storage.
Despite these advantages, challenges still arise, similar to those when developing liquid formulations. For example, particle size and distribution remain vital to dose uniformity, which ensures that particles are deposited and dispersed with consistency, avoiding the vestibules. Particle size and distribution are also critical to ensure delivery of the dry powder into the turbinates, olfactory and nasopharynx regions while avoiding delivery downstream into the lungs. Device design also plays an essential role, requiring the powder to be retained in a protected environment prior to administration while ensuring that the aerodynamic properties of the delivered powder optimise deposition within the nasal cavity.
In addition to meeting the TPP, the drug product properties must also fulfil regulatory requirements to ensure safety and efficacy. In particular, aerodynamic particle size restrictions are enforced to prevent delivery of the drug product into the lung as opposed to remaining in the nasal passage.
A ROBUST PLATFORM IS KEY TO OVERCOMING CHALLENGES AND MITIGATING RISK
Employing a robust platform strategy can help to identify potential problems early when creating a drug product for nasal delivery, enabling solutions to be implemented pre-emptively to accelerate overall development and manufacture. In addition to helping to overcome challenges, an effective platform strategy can mitigate production risks. A robust platform strategy should encompass the following critical steps:
“To meet these growing demands, innovation in the nasal delivery space is essential for producing drug products that meet stability, solubility and bioavailability standards.”
- TPP development: Identify the desired characteristics of the product (such as anticipated dose and frequency of dosing).
- Pre-formulation/stability screening: Complete stability testing studies throughout in a liquid or dry powder formulation, including excipient compatibility and accelerated stability testing.
- Selection of dosage form: Use the screening results to decide upon a dosage form (such as liquid vs dry powder or single shot vs multidose).
- Formulation development: Determine the final formulation and process following regulatory submissions and toxicology evaluation prior to clinical manufacture.
Irrespective of whether the product is to be a liquid or dry powder formulation, an effective platform strategy can help to inform key decisions, with early identification of drug product needs allowing for proactive development and manufacture. With a platform strategy helping to mitigate risk and streamline the development of the final formulation, the clinical dosage batch can then be manufactured and the device filled.
SOLIDIFYING THE FUTURE OF NASAL DELIVERY DEVICES
The pharmaceutical industry is showing an increased interest in nasal delivery as an alternative needle-free option for the self-administration and rapid delivery of drugs into systemic circulation and to the brain. To meet these growing demands, innovation in the nasal delivery space is essential for producing drug products that meet stability, solubility and bioavailability standards. Researchers are increasingly looking at the development of dry powder nasal dosage forms to overcome these challenges. Despite this growing interest in dry powder nasal drug delivery, understanding of the steps necessary for developing nasal products remains limited.
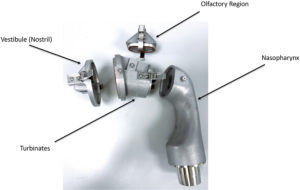
Figure 1: Parts of an AINI.
Challenges remain not only in the development of nasal dosage forms but also in the identification of analytical methods suitable to testing their performance. During the development stages and when the final prototype dosage form has been finalised, analytical test methods are essential for ensuring that the final dosage form is stable and exhibits the correct delivery performance.
One key area of research is in the development of relevant tests to determine the aerodynamic performance and nasal deposition patterns for nasally delivered drugs. This area is attracting significant interest from developers and regulators alike, who want to know how much of the dose is delivered from the device as well as where it is deposited in the nose, and potentially in the lungs. This has led to the development of next-generation nasal deposition models, such as the Alberta idealised nasal inlet (AINI), shown in Figures 1 and 2.
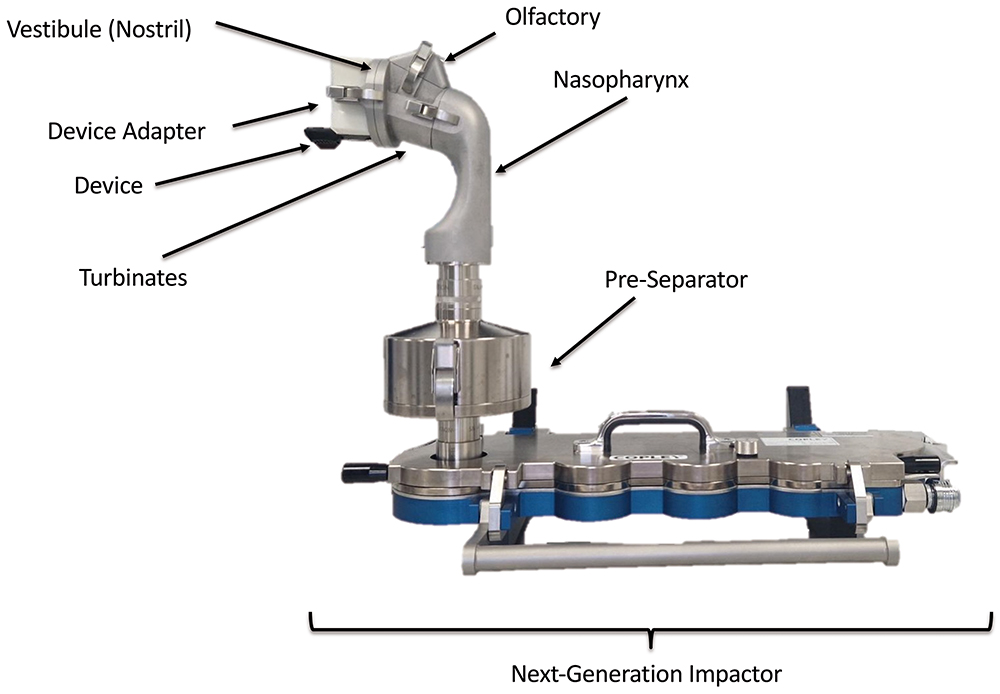
Figure 2: AINI fixed to Copley Scientific (Nottingham, UK) next-generation impactor for aerodynamic particle size distribution and lung deposition analysis.
A partnership with an expert in nasal manufacture and delivery can help to provide specialist technical and regulatory knowledge that can drive a nasal drug product to success. Starting a collaboration early in development allows for the TPP to be outlined and the drug product formulated and filled with the desired use in mind, accurately addressing the needs of the product and the patient.

