Citation: “Next-Generation Capsule Filling: MG2 and the DM2 Project.” ONdrugDelivery Online, July 26, 2022.
MG2 introduces the UK Made Smarter Innovation – Digital Medicine Manufacturing (DM²) Research Centre, which it has recently joined as an industrial partner, and describes how it will develop an AI-driven microscale capsule filling system for the selection of formulation and process parameters.
MG2 has joined the UK’s “Made Smarter” movement by participating as an industrial partner in its Digital Medicine Manufacturing (DM²) Research Centre (Figure 1).

Figure 1: DM² aims to advance medicines development, manufacture, quality control (QC) and supply via data-driven industrial digital technologies such as AI, robotics and digital twins.
DM², which is led by CMAC (Continuous Manufacturing & Advanced Crystallisation) at the University of Strathclyde (Glasgow, UK), in partnership with Loughborough (UK) and Cambridge (UK) universities, aims to advance medicines development, manufacture, quality control (QC) and supply through the creation and accelerated adoption of data-driven industrial digital technologies (IDTs) including AI, robotics and digital twins.
“MG2 is primarily involved in Platform 2, which will create an AI-driven microscale capsule filling system to select formulation and process parameters that result in a stable product with the desired critical quality attributes (CQAs).”
FIVE INTEGRATED RESEARCH PLATFORMS
To achieve these goals the work is being delivered across five integrated research platforms:
- Platform 1: The Data Platform
- Platform 2: Autonomous Microscale Manufacturing
- Platform 3: Digital Quality Control
- Platform 4: Adaptive Digital Supply
- Platform 5: DM² Network & Skills.
MG2 is primarily involved in Platform 2, which will create an AI-driven microscale capsule filling system to select formulation and process parameters that result in a stable product with the desired critical quality attributes (CQAs).
IMPROVING ON THE STATUS QUO
The current practice in drug development presents significant challenges in terms of the number of experiments drastically increasing with the number of excipients, drug loadings and process conditions that need to be explored to find the optimal formulation and process settings. IDTs with innovative pharmaceutical process technologies will de-risk and accelerate drug product development and manufacture, reducing experiments whilst achieving CQA objectives (Figure 2). An initial aim for this project is to digitalise the capsule filling processes using hybrid models with predictive power that can inform the decision-making process.
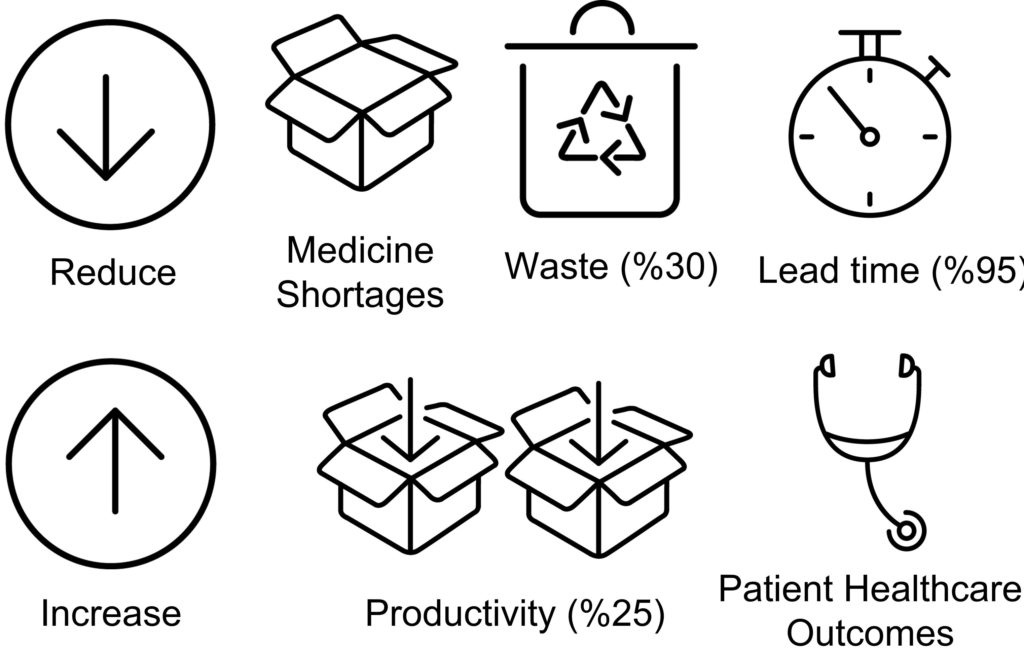
Figure 2: Scope of the project.
The objectives of Platform 2 are two-fold:
- To develop a digital twin of the capsule filling process
- To create automated Design of Experiment (DoE) that enables autonomous process optimisation through AI-driven experimentation.
The objectives will be achieved through the following operational steps:
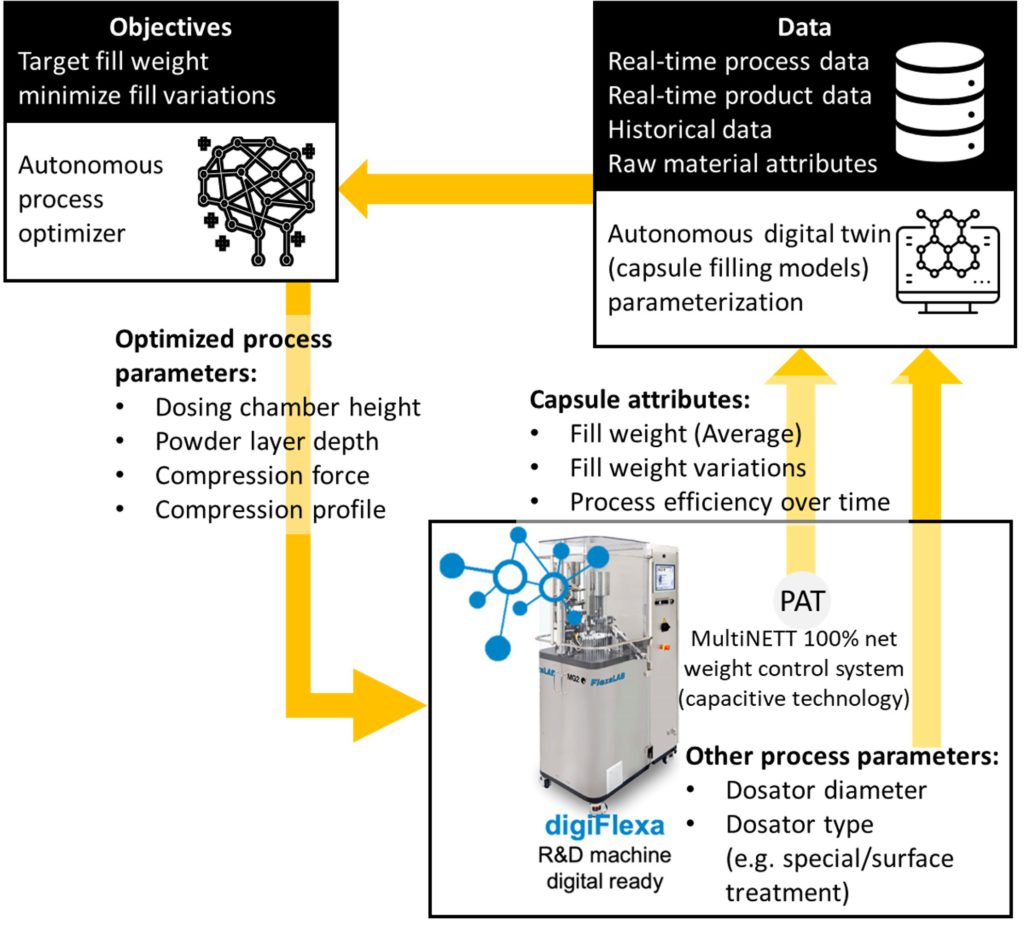
Figure 3: Capsule filling hybrid modelling
-
- Training on the FlexaLAB capsule filler machine at MG2 headquarters.
- Installation of a FlexaLAB capsule filler at the University of Strathclyde (CMAC).
- Experimental data collected using FlexaLAB to develop the initial models (Figure 3) to target fill weight and the process efficiency over time.
- Digitalisation of FlexaLAB (digiFlexa)
upon the completion of steps one,
two and three (Figure 4).
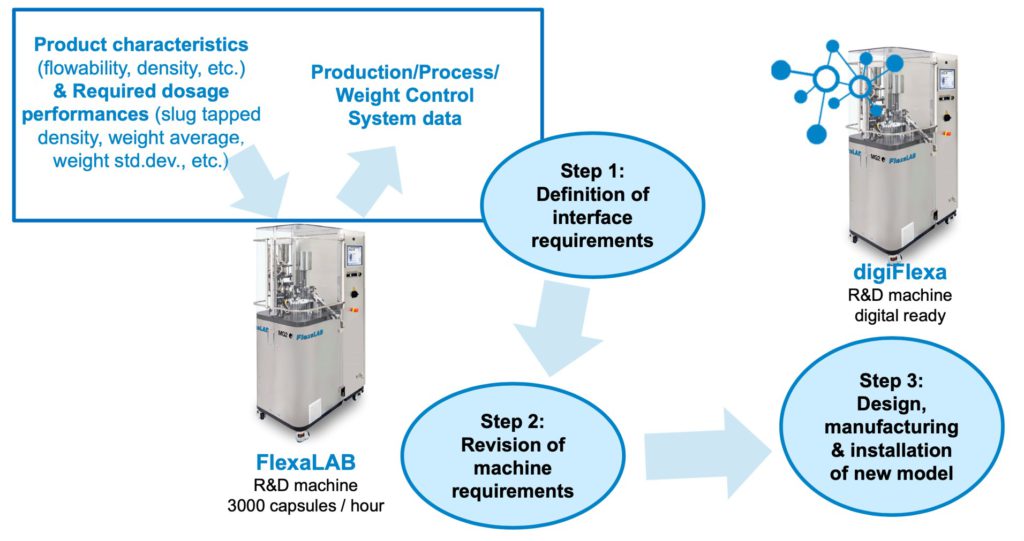
Figure 4: Schematic representation of digitalisation workflow for FlexaLAB capsule filling machine
DIGIFLEXA: DIGITISATION OF FLEXALAB
The initial version of digiFlexa will feature hybrid modelling of the fill weight and the fill weight variability. This feature will then be integrated into a numerical decision-making framework to autonomously optimise the process conditions (for a given formulation) such as the dosing chamber height, powder layer depth, compression force and profile. At the next stage, the automated process optimisation will be followed by the formulation setting optimisation.
The overarching goal aligns with CMAC’s industry-led strategy for digitalisation of CMC and is to maximise the value of information extracted from measurements while minimising material consumption in order both to make the right formulation and test the right property at the right time. To do this, it is necessary to evolve the FlexaLAB capsule filling machine to a digital-based platform with associated workflow that enables users to utilise the tools quickly and implement the digitalised capsule filling system (digiFlexa) to control and optimise CQAs of capsules by design.
For more information on this exciting new research, contact Platform 2 Academic Lead Dr Daniel Markl ([email protected]), or Industry Engagement Lead Massimo Bresciani ([email protected]). Follow the project’s progress on the DM2 website, and via its LinkedIn and Twitter channels.
Previous article
ROUNDTABLE: TRENDS AND INNOVATIONS IN ORAL DRUG DELIVERYNext article
INTERVIEW: Piyush Agarwal, Tjoapack
