To Issue 137
Citation: Oakley T, “On-Body Delivery Systems – News and Trends”. ONdrugDelivery, Issue 137 (Sep 2022), pp 12–16.
Tom Oakley discusses the current state of play in the on-body delivery system space, reviews recent milestones and proposes directions for the future.
“OBDSs can be used for a wide, ever-increasing range of indications and drugs, particularly those where the relatively high volume, viscosity or dose timing makes autoinjectors inappropriate.”
INTRODUCTION
The on-body delivery system (OBDS) market is changing rapidly and it has become clear that these devices are critical to the delivery of some new drugs that are currently in development. The OBDS market is relatively young, which lends itself to exciting innovations and a high potential growth rate.
Before launching into the details, it is important first to clarify which OBDSs are under discussion. By far the largest market for wearable injectors is diabetes; however, ambulatory insulin infusion pumps will be considered out of scope of this article because they deserve an article all to themselves. They are focused on one therapy where the flowrate is clinically relevant and their requirements are covered by IEC-60601-2-24 (if electronic) or ISO 28620 (if non-electronic).
For this article, the focus will be on OBDSs where the flowrate is not clinically relevant (other than avoiding the flowrate being too high, which could cause patient discomfort or a leak) and their requirements are covered by ISO 11608-6:2022. Such OBDSs can be used for a wide, ever-increasing range of indications and drugs, particularly those where the relatively high volume, viscosity or dose timing makes autoinjectors inappropriate. Examples include pegfilgrastim, for reducing infections after chemotherapy; evolocumab, for reducing cholesterol; and various drugs for Parkinson’s disease.
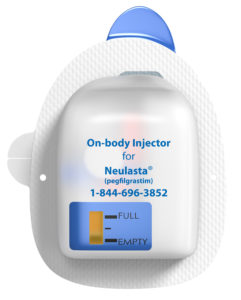
Figure 1: Amgen’s Neulasta.
RECENT MILESTONES
The first launch of the modern generation of non-insulin OBDSs was Insulet’s (MA, US) Onpro® for Amgen’s Neulasta® drug in 2015 (Figure 1).1 This is a modified version of the Omnipod insulin pump and delivers the injection in one bolus approximately 27 hours after the device is filled from a prefilled syringe by a healthcare professional and attached to the patient’s body. Since its launch, over one million patients have used the Onpro OBDS to deliver Neulasta.2 The drive mechanism uses shape memory alloy (“muscle wire”) to reciprocate a lever arm, which rotates a ratchet wheel and leadscrew to push the plunger. The drug container is a custom oval plastic cartridge.
West’s SmartDose 3.5 mL (Figure 2) was launched in 2016 for Amgen’s Repatha® and branded “Pushtronex®”.3 In 2019, scPharmaceuticals (MA, US) announced its intent to go to market with West’s SmartDose® 10 injector for FUROSCIX®, a proprietary subcutaneously delivered furosemide solution for the treatment of worsening heart failure due to congestion.4 The platform uses an innovative telescopic plunger rod to minimise device size. The primary drug container is a custom cartridge made of cyclo-olefin polymer.
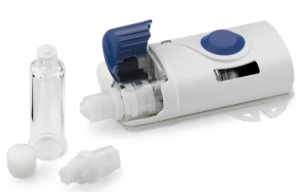
Figure 2: West’s SmartDose 3.5 mL.

Figure 3: Enable’s EnFuse.
Enable Injections has supported three clinical studies using its enFuse® pump (Figure 3), one of which is published.5 Pharmaceutical partners can use their original drug container because the user fills the device just before use, thus inflating an elongated elastomeric drug container. Once on the body and activated, the elastic energy of the drug container expels the drug formulation in “bands” to smooth out the pressure over time. Enable has announced relationships with Lilly, Sanofi, Apellis, Genentech, UCB and CSL Behring, and has received US$215 million (£178 million) in financing as of January 2022.6
Ypsomed’s YpsoDose single-use injector is an electromechanical prefilled and pre-assembled patch device for 10 mL glass cartridges (Figure 4). Needle insertion, injection, end of injection feedback and needle safety steps are all performed automatically. The needle remains hidden at all times and is made safe after injection and device removal. Ypsomed is scaling up its manufacturing capability for YpsoDose in Switzerland with first clinical studies starting in 2023.

Figure 4: Ypsomed’s YpsoDose.
Nemera has completed a formative study on its Symbioze wearable pump (Figure 5) and expects to have new prototypes from rapid tooling by the end of 2022. The design has a reusable subassembly containing the motor and electronics, alongside a disposable subassembly containing the drug path and container. The reusable subassembly checks the identification of the disposable subassembly using near-field communication (NFC) before injection. The reusable electronics allow for additional features with minimal impact on cost or the environment, such as Bluetooth connectivity.

Figure 5: Nemera’s Symbioze.
“Pharmaceutical companies continue to see the drug container as a key risk, especially for prefilled devices, because anything that is not a standard glass pharmaceutical cartridge requires a lot more risk management and evidence before being accepted by regulators.”
BD Medical – Pharmaceutical Systems has announced two OBDS platforms that are in development:
- BD Evolve™ On-body Injector is a programmable platform supporting injections up to 3 mL. BD has conducted nine pre-clinical and five human factor studies
- BD Libertas™ Wearable Injector supports 2–5 mL or 5–10 mL doses in its two sizes and is fully mechanical (no electronics or software). The drug container is a nearly standard glass cylinder, available in standard nest and tub configurations. In 2021, BD published a clinical study on the 5 mL variant with 52 subjects to test functionality and acceptability with patients.7
Gerresheimer (Düsseldorf, Germany) acquired Sensile Medical and now has three OBDSs in its offering:
- SensAir, a pump optimised for delivering large molecules (Figure 6)
- An OBDS based on the SenseCore rotating piston pump for small molecules
- A belt-worn delivery system, currently used by EVER pharma to deliver apomorphine for Parkinson’s disease.
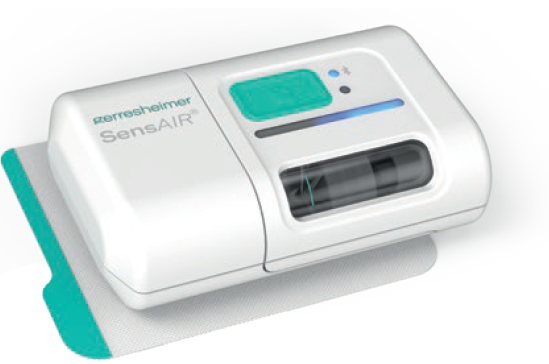
Figure 6: Gerresheimer’s SensAir.
Bespak, now owned by Recipharm, is not actively developing its hydrofluoroalkane-powered Lapas wearable injector. Similarly, SteadyMed was acquired by United Therapeutics (MD, US), which took the expanding-battery-driven Patch Pump® out of the running.
CURRENT DEVICE STRATEGIES
Early in development, the team must choose between using a standard or custom drug container. There is frequent temptation to use a custom drug container to improve device layout, enable novel delivery mechanisms and improve usability. As such, it is no surprise that there are numerous OBDSs in development with custom drug containers, and many more that have been discontinued.
However, pharmaceutical companies continue to see the drug container as a key risk, especially for prefilled devices, because anything that is not a standard glass pharmaceutical cartridge requires a lot more risk management and evidence before being accepted by regulators. The choice of drug container is particularly important for prefilled devices because they typically have strict requirements for drug stability over two years’ shelf life.
Secondly, the device designer must choose between two fundamental methods of delivering the drug to the patient – either pushing the drug out of the container or pulling the drug out of it.
| Method | Advantages |
| Push drug out of container |
|
| Pull drug out of container |
|
Table 1: Advantages of pushing or pulling the drug out of its container.
Table 1 lists the advantages of each method; note that the advantages of one method mirror the disadvantages of the other, so listing the disadvantages is unnecessary. Table 2 shows how different companies are providing OBDSs with different strategies with respect to “push” or “pull”.
| Method | Standard or near standard drug container | Non-standard drug container |
| Push drug out of container |
|
|
| Pull drug out of container |
|
|
Table 2: Publicly announced pumps categorised by push/pull and drug container.
“The OBDS market is in an exciting and pivotal phase, with some devices on the market, many more devices racing to clinical studies and some falling by the wayside.”
NEW ISO 11608-6 STANDARD
The ISO 11608 family of international standards has been recently updated, with many published in April 2022. ISO 11608 Part 6 is new and covers OBDSs, specifically covering fixed-dose delivery systems where the medicinal effect is measured by the dose volume, not the flowrate. In contrast, delivery systems that are designed to deliver given flowrates are covered by IEC 60601-2-24 and ISO 28620. The standard is designed to cover requirements that are not typically present for handheld injection devices, such as:
- Means of attachments to the body
- Occlusion detection
- Leakage due to worst-case environments, and orientation
- Changes in or periods with vibration, temperature, humidity, atmospheric pressure, light exposure and orientation whilst in use
- Dose delivery over time.
It also defines test methods for adhesion, dose delivery profiles and needle/cannula displacement whilst in the body.
The additional requirements described in the 11608-6 standard should make it clearer to stakeholders why there can be more development work required to bring an OBDS to market compared with a prefilled syringe. At the same time, the clarity brought by the standard should reduce the time that device developers and regulatory affairs people spend trying to work out the requirements and expectations for new OBDSs.
CHALLENGES
Considerable progress has been made on the main challenges for OBDSs in recent years. For example, while many patients, caregivers and healthcare professionals are familiar with autoinjectors, the use scenarios of OBDSs are unfamiliar. As such, the occurrence of certain usability risks could be increased. To combat this, usability (human factors) now needs to be considered early and throughout device development according to the two main regulatory requirements in the area:
- US FDA guidance “Applying Human Factors and Usability Engineering to Medical Devices”
- IEC 62366-1:2015 “Application of usability engineering to medical devices”.
However, there are still some companies that prefer not to conduct early formative human factors studies, despite them being critical to determining a meaningful user requirements specification.
Another challenge where progress has been made is the need for parenteral injections to be sterile. The sterility strategy for prefilled syringes tends to be straightforward – the syringe is sterilised with its needle shield in place then filled in a sterile isolator. However, most OBDSs do not use a prefilled syringe as a primary container and therefore need a strategy to both achieve and maintain sterility. This can require some novelty when the drug container is not connected to the needle or cannula before use because there could be a non-sterile region between those two subassemblies. With suitable design, various methods for sterilising the drug path have been used successfully in different devices, such as ethylene oxide, nitrogen dioxide, ultraviolet radiation in situ and others.
That said, three challenges have continued unabated and may even have increased recently. First, cold chain distribution presents a challenge to device developers. Most biologics, which are often the target for OBDSs, need to be transported and stored at refrigerated temperatures. Therefore, if the OBDS is prefilled, it must be robust to cold temperatures and condensation, which can be challenging for devices containing electronics and batteries. In addition, the overall size of the device and its packaging must be minimised because cold chain space is finite, expensive and has a significant environmental footprint.
Second, demands from patients, payers and pharmaceutical companies to reduce environmental impact are increasing. Some devices have been selected on their environmental credentials. Compared with prefilled syringes or autoinjectors, wearable injectors tend to:
- Require more plastic
- Need an adhesive patch
- Need soft cannula insertion (and perhaps retraction)
- Need electronics and batteries.
To address this, some wearable injectors employ strategies such as:
- Splitting the device into reusable and disposable subassemblies
- Increasing the number of possible times a patient can reuse a given device
- Collecting a used device from one patient, reconditioning it and sending it to another patient to use.
Thirdly, the intellectual property space around OBDSs has become increasingly crowded. As such, freedom to operate is a key issue.
FUTURE DIRECTIONS
It is possible to speculate on future directions of development for OBDSs. Looking forward, the major themes are likely to be:
- Rationalisation of requirements and designs. That is, requirements and designs will simplify according to which user experiences are discovered to be most successful on the market and, in turn, which user experiences become expected by patients, carers, healthcare professionals and payers. For example, autoinjectors seem to have moved from “automatic needle insertion with button activation” to “manual needle insertion with push-onto-body activation”. These changes may lead to simpler and smaller devices
- Tension between adding functionality, such as connectivity, versus optimising for environmental sustainability
- Connection with companion diagnostics. This will be the subject of a panel discussion at the Partnerships on Drug Delivery (PODD) conference in Boston in October 2022
- In time, rationalisation of wearable device suppliers in the marketplace. As a relatively new device category, there is a lot of innovation and competition. Like any other disruptive industry or device category, a handful of players may come to dominate the market over the coming years because pharmaceutical customers may prefer proven technologies, tooling and production costs might become amortised for devices already on the market, and some device manufacturers will gain early-mover advantage.
SUMMARY
More than at any other time, in 2022, the world recognises the need for high-quality, accessible and sustainable healthcare. The OBDS market is in an exciting and pivotal phase, with some devices on the market, many more devices racing to clinical studies and some falling by the wayside. However, pressures from environmental sustainability, ever-increasing usability requirements and the demand for new features mean that the race is far from over.
If you have questions or would like to discuss any points, please do not hesitate to contact the author.
REFERENCES
- Amgen Announces Launch Of New Neulasta® (Pegfilgrastim) Delivery Kit”. Press Release, Amgen, Mar 2, 2015.
- “Neulasta® Onpro® helps you fight the risk of infection from home and reduces your risk of being hospitalized”. Patient Brochure, Neulasta®, 2020.
- “West’s SmartDose® Drug Delivery Technology Platform Selected by Amgen for Pushtronex™ System”. Drug Development & Delivery, Jul 2016.
- Patel A, “The Path to Commercialisation for Wearable Drug Delivery Devices”. ONdrugDelivery, Issue 124 (Sep 2021), pp 58–62.
- “Sanofi announces €300 million collaboration with Blackstone Life Sciences to advance an innovative treatment for multiple myeloma”. Press Release, Sanofi, Mar 15, 2022.
- “Enable Injections Announces $215 Million Financing”. Press Release, Enable Injections, Jan 27, 2022.
- Woodley WD et al, “Clinical Evaluation of an Investigational 5 mL Wearable Injector in Healthy Human Subjects”. Clin Transl Sci, 2021, Vol 14(3), pp 859–869.

