To Issue 129
Citation: Sakuma Y, Suzuki T, “Photo-Stability Tests of Epinephrine”. ONdrugDelivery Issue 129 (Feb 2022), pp 64–68.
Yoshiko Sakuma and Tomohiro Suzuki provide an overview of OXYCAPT multilayer vial and syringe, with a particular emphasis on the advantages of OXYCAPT as a container for adrenaline, as demonstrated by recent studies conducted by MGC.
Mitsubishi Gas Chemical (MGC) is a leading company in the field of oxygen barrier and absorbing polymer technologies. Building on these technologies and experiences, MGC launched OXYCAPT™, a new multilayer plastic vial and syringe (Figure 1), in 2019. The material consists of three layers – the drug contact layer and the outer layer are made of cyclo-olefin polymer (COP), and the oxygen barrier layer is made of MGC’s novel polyester (Figure 2). OXYCAPT offers many advantageous properties, including:
- Excellent oxygen and ultraviolet (UV) light barrier
- Strong water vapour barrier
- Very low extractables
- High pH stability
- Low protein adsorption and aggregation
- Silicone-oil-free barrel
- High transparency
- High break resistance
- Easy disposability
- Lightweight.

Figure 1: The OXYCAPT multilayer plastic vial and syringe.
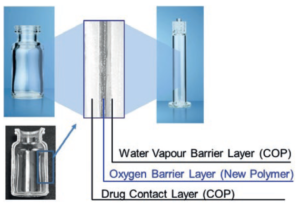
Figure 2: Multilayer structure of OXYCAPT.
MGC has conducted a series of studies to confirm these excellent properties. This article will provide both a more detailed overview of OXYCAPT’s properties and insight into a study focusing on the photostability of adrenaline in OXYCAPT, Type 1 glass and COP.
PROPERTIES OF OXYCAPT VIAL & SYRINGE
There are two types of OXYCAPT multilayer plastic vial and syringe – OXYCAPT-A and OXYCAPT-P. OXYCAPT-A offers a glass-like oxygen barrier. According to internal studies, thanks to its oxygen-absorbing function, OXYCAPT-A can maintain lower oxygen concentrations in the headspace than Type 1 glass. OXYCAPT-P also provides an excellent oxygen barrier, although there is no oxygen-absorbing function. For example, the oxygen barrier of an OXYCAPT-P vial is about 20 times better than that of a COP monolayer vial (Figure 3).
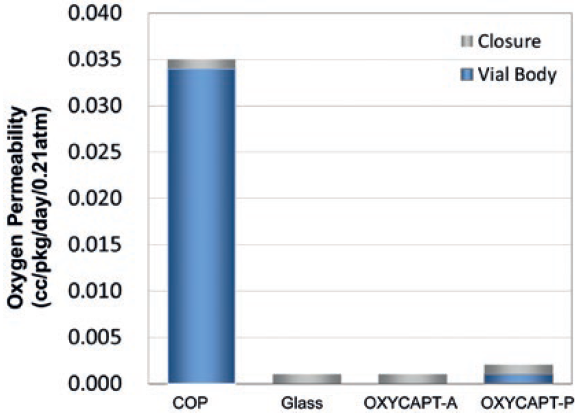
Figure 3: Oxygen permeability comparison of a typical COP, glass, OXYCAPT-A
and OXYCAPT-P.
OXYCAPT also provides an excellent UV barrier. While about 70% of 300 nm UV light transmits through glass and COP, only 1.7% transmits through OXYCAPT (Figure 4). MGC has confirmed that this feature contributes to the stability of biologics.
While OXYCAPT cannot reach the performance of glass with respect to acting as a water vapour barrier, its properties are similar to those of COP, which has been used for injectable drugs for a long time. This means that OXYCAPT easily meets the requirements of a water vapour barrier set out by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines.
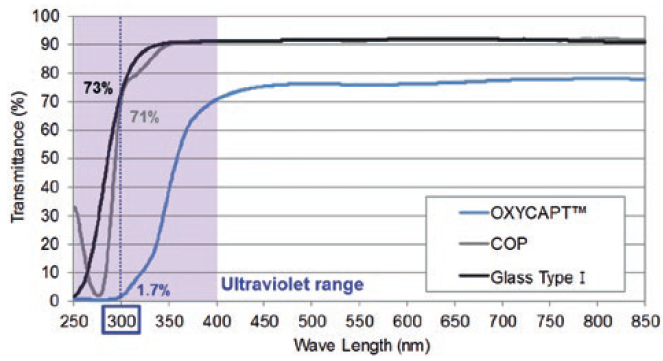
Figure 4: UV light transmittance comparison of a typical COP, Type 1 glass and OXYCAPT.
Studies have shown an extremely low level of extractables from OXYCAPT. One study was conducted to confirm the levels of volatile, semi-volatile and non-volatile impurities from OXYCAPT. Water and four solutions (50% ethanol, NaCl, NaOH and H3PO4) were selected, and impurities were measured by gas chromatography mass spectrometry (GC-MS) and liquid chromatography-UV spectroscopy-mass spectrometry (LC-UV-MS) after 70 days at 40°C. Compared with the control, impurities were not detected in the OXYCAPT containers. A second study confirmed that inorganic extractables levels from OXYCAPT were similar to those from COP, which is well known for being an extremely pure polymer, and with a better extractables profile than Type 1 glass. Lower levels of inorganic extractables are known to contribute to better pH stability in drug products.
The OXYCAPT vial and syringe is produced by co-injection moulding technology. Although this technology has been used in the production of beverage bottles for many years, MGC is the first company to succeed in applying it to the production of multilayer plastic syringes.
MGC has also developed inspection methods for testing the oxygen barrier layer. All of the containers are fully inspected by state-of-the-art inspection machinery. MGC can offer bulk vials, ready-to-use (RTU) vials and RTU syringes. Regarding the RTU products, vials and syringes are provided in ISO-based nest and tub formats. The nest and tub are mainly sterilised using gamma rays. There are 2, 6, 10 and 20 mL variants for vials, and 1 mL long and 2.25 mL variants for syringes (Table 1). MGC is willing to provide samples for initial testing free of charge.
| Type | Volume | ISO | Parts | Option |
| Vial | 2 mL | ISO 8362-1 | Vial | Bulk or RTU |
| 6 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| 10 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| 20 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| Syringe | 1 mL long | ISO 11040-6 | Barrel, Tip Cap, Stopper, Plunger Rod |
RTU |
| 2.25 mL | ISO 11040-6 | Barrel, Tip Cap, Stopper, Plunger Rod |
RTU |
Table 1: MGC’s OXYCAPT product portfolio.
Each polymer meets the requirements of United States Pharmacopeia (USP) regulations USP<661>, USP<87> and USP<88>, as well as those of the European Pharmacopeia, and has been filed in the US FDA’s drug master file (DMF). The vials and syringes are also compliant with each pharmacopoeia and have been filed in the DMF. The syringes are produced and controlled in accordance with ISO 13485.
OXCAPT’S SUITABILITY FOR ADRENALINE
The primary target market for OXCAPT is the therapeutic application of biologics. As mentioned in ICH Q5C (Stability of Biotechnological/Biological Products), oxidation is one of the causes of protein instability. As such, the oxygen and UV barrier properties of OXYCAPT will contribute to the stability of biologics stored within. Additionally, MGC believes that OXYCAPT is well suited to emergency adrenaline, which is well known as an oxygen-sensitive drug, because OXYCAPT combines both an oxygen barrier equivalent to Type 1 glass and the breakage resistance of a polymer. Furthermore, some drug developers have recently started evaluating the OXYCAPT vials for their gene and cell therapies; the RTU vial is sterilised by gamma radiation, making it ideal for protein-based drugs.
In order to verify the suitability of OXYCAPT for this purpose, photostability tests on commercially available adrenaline drug products were carried out using OXYCAPT, Type 1 glass and COP containers. In this study, OXYCAPT-P, Type 1 glass and COP vials and syringes were filled with 1 mL preparations of 0.1 mg/mL adrenaline under nitrogen atmosphere. The preparations were stored under light at 4,000 lux/hour at 40°C for 12.5 days, resulting in a total radiation dose of 1.2 million lux.
After exposure to radiation, the investigation examined colour change and residual adrenaline in the preparations. The colour change was evaluated by visual inspection and the residual adrenaline was evaluated by ultra performance liquid chromatography (UPLC). The impurities generated by oxidation of epinephrine were identified by liquid chromatograph time of- flight mass spectrometry (LC-TOF-MS). Commercially available adrenaline that had not been exposed to light was used as a control.
Although no colour change was observed in OXYCAPT™ and Type 1 glass containers, browning was observed in the adrenaline contained in COP. As the oxygen and ultraviolet barrier properties of COP containers is known to be very poor, it can be assumed that the UPLC in COP deteriorated as a result of oxygen and UV light (Table 2).
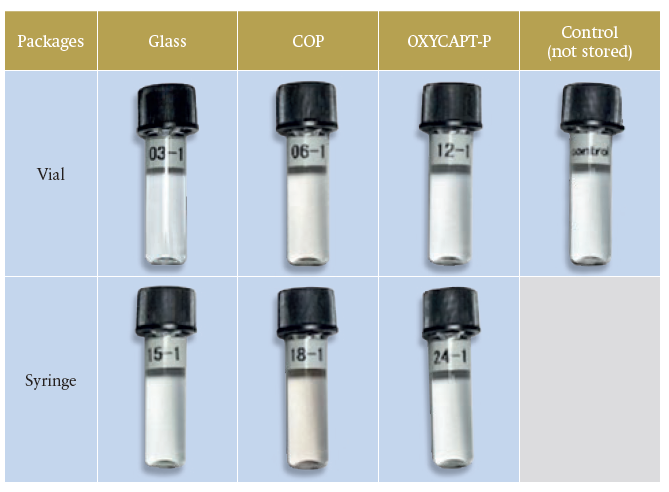
Table 2: Visual inspection of adrenaline.
UPLC analysis showed that no significant difference in adrenaline concentration was observed between each container compared with the control and that the rate of residual adrenaline was 98–102% (Figure 5). Although the colour of the adrenaline in COP containers turned brown, no significant difference in the quantity of residual epinephrine was observed by UPLC analysis. Therefore, it can be assumed that the adrenaline in COP containers turned brown due to slight oxidation.
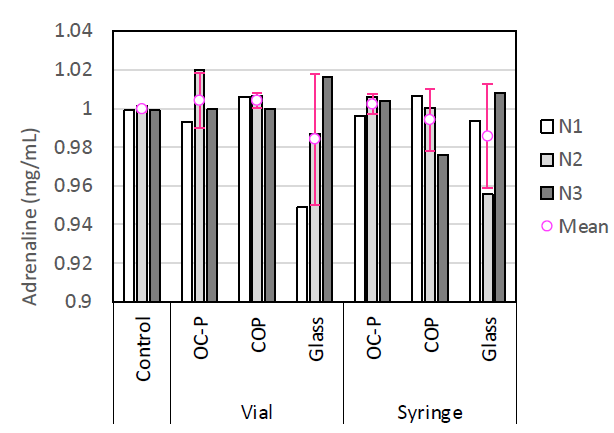
Figure 5: Concentration of adrenaline.
Additionally, some peaks representing impurities were found on the chromatographs of all the containers that were not detected in that of the control (Figures 6 & 7). The area of the COP vial’s peaks was the largest and that of Type 1 glass the smallest (Table 3). By LC-TOF-MS analysis, the impurities were identified as adrenaline sulfate, oxo-adrenochrome, adrenochrome and leuko-oxo-adrenochrome. Furthermore, a high level of methyl-substituted adrenaline-o-quinone was detected.
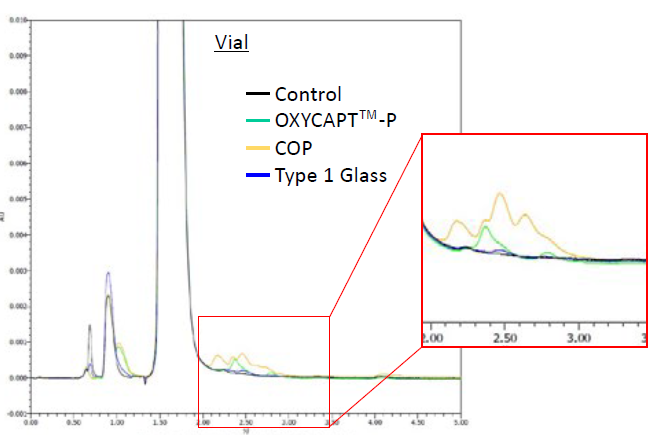
Figure 6: Impurities recognised on a chromatogram of vials.
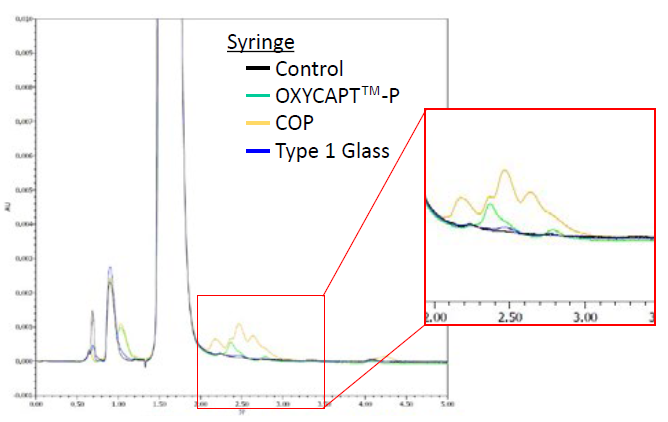
Figure 7: Impurities recognised on a chromatogram of syringes.
| Container | Material | Ratio of impurity’s peak area (%)1 |
| Vial | OXYCAPT-P | 0.15 |
| COP | 0.43 | |
| Glass | cannot calculate2 | |
| Syringe | OXYCAPT-P | 0.16 |
| COP | 1.38 | |
| Glass | cannot calculate2 |
1 N = 3, RT = 2–3 min,2 Peak was too small to calculate
Table 3: Impurity ratios of adrenaline preparations after exposure to light.
CONCLUSION
These latest results have contributed to the ongoing studies verifying OXYCAPT’s superior properties for containing biologics, and adrenaline in particular. In addition to the advantages of COP, such as a strong water vapour barrier, high break resistance, very low extractables and low protein adsorption, OXYCAPT also provides a strong oxygen and UV light barrier. MGC believes that OXYCAPT offers a multitude of benefits to the rapidly growing field of biologics and gene/cell therapies.

