Citation: Green J, “Plasma Technology – the Future of Respiratory Devices”. ONdrugDelivery, Issue 126 (Oct/Nov 2021), pp 57–60.
Jacqueline Green discusses how H&T Presspart’s plasma treatment process can provide a future-proof and sustainable solution for some of the issues associated with suspension formulations in the respiratory device industry.
“To meet both the demands of sustainability and MDI canister design, H&T Presspart has developed a proprietary plasma treatment process that provides a sustainable, future-proof and cost-effective solution.”
Metered dose inhaler (MDI) formulations typically exhibit two main failure modes: drug degradation and drug adhesion, both of which occur as a result of the formulation interacting with the metal canister. In order to address these issues, a matrix of canister solutions has been established over time.
However, there is an increasing focus on sustainability across all industries and sectors of society, which has led to an imperative for more sustainable drug delivery solutions. In this context, the majority of these alternative cans may fall short of expectations, due to increasing environmental regulations and cost pressures.
H&T Presspart’s plasma treatment process that provides a sustainable, future-proof and cost-effective solution. This process addresses the main issues encountered with formulations in the industry, with its ever-challenging and increasingly complex molecules, combinations and propellants.
FORMULATION CHALLENGES
“In order to prevent or reduce drug degradation, canisters need to provide a barrier between the exposed aluminium on the interior of the can and the formulation.”
H&T Presspart has a 50-year history of manufacturing aluminium canisters for pressurised MDIs (pMDIs). This experience has informed the company’s efforts to tackle the two main failure modes that some formulations can exhibit. As mentioned prior, the first of which is drug adhesion, where drug sticks to the internal surfaces of the canister. This failure mode is encountered specifically in suspension formulations.1,2
The second is drug degradation, where increased impurity levels are observed as the drug deteriorates over time due to interactions with natural oxides and organic residues present on the internal surfaces of the canister. This is evident in solution formulations and reduces the shelf life of the product as a result. Both of these failure modes result in reduced drug content, meaning that the patient can receive less than the prescribed dose.
With increasingly complex formulations and molecules being developed, as well as improvements in propellants and the need for more sustainable, future-proof solutions, the role of the canister has transformed from merely providing safe containment for the drug product to being an integral part of the drug delivery system itself, increasing the importance of drug-canister interfaces.
TRADITIONAL COATINGS AND SOLUTIONS
In order to prevent or reduce drug degradation, canisters need to provide a barrier between the exposed aluminium on the interior of the can and the formulation. One solution is to use an alternative substrate – stainless steel, rather than aluminium – for more aggressive formulations. Another solution is to anodise the aluminium cans, removing the natural oxides and organic residues and replacing them with a structured anodised aluminium layer.

Figure 1: H&T Presspart’s automated MDI plasma canister manufacturing cell.
However, in most cases, these solutions are only useful for solution-type formulations, and do not address adhesion issues. Furthermore, anodising only slows degradation, rather than preventing it. There is also an increased cost involved with these kinds of cans due to the more expensive stainless steel material and the anodising process.
An alternative solution is spray coating with fluorinated ethylene propylene (FEP), which provides both a barrier and low surface energy. However, once again, this solution presents cost and sustainability issues. With all this in mind, Presspart partnered with Portal Medical (Cambridge, UK) to develop a solution that addresses both aggregation and degradation, but also provides superior performance characteristics at a lower variable cost than the alternatives, with no supply or legislative barriers, and freedom to operate across all drug candidates in all markets. This solution is Presspart’s patented plasma canister (Figure 1).
PLASMA TECHNOLOGY
In general terms, plasma is known as the fourth state of matter. Solids plus energy produce liquids. If energy is added to liquids, they produce gases. Plasma, an ionised gas, is produced when radio frequency energy is applied to free electrons in the gas causing ions and electrons to co-exist, which creates performance surfaces whilst maintaining bulk strength properties.3 Plasma, or fluorocarbon polymer (FCP), is a nanolayer on the internal surface of the canister. This is different to traditional coatings, as plasma is not a substance applied directly onto the interior can surface – it is a change to the molecular structure of the canister interior, covalently, and inseparably, bonded to the aluminium surface. With the treatment limited to the internal surface of the can, label adhesion and filling line performance are not compromised.
Budesonide Study – An Example
The chemical stability of a 50 μg budesonide solution formulation contained within five alternative canisters was tested and compared. Figure 2 shows the results following 1-month (40°C/75% RH) and 3-month (40°C/75% RH) storage (valve-up and valve-down).
For the budesonide solution study, surface-treated canisters outperformed stainless steel and plain aluminium canisters, showing high integrity and an inert relationship with the formulation and, therefore, less degradation with the treated can types. This was the case for both the initial and 3-month time points.
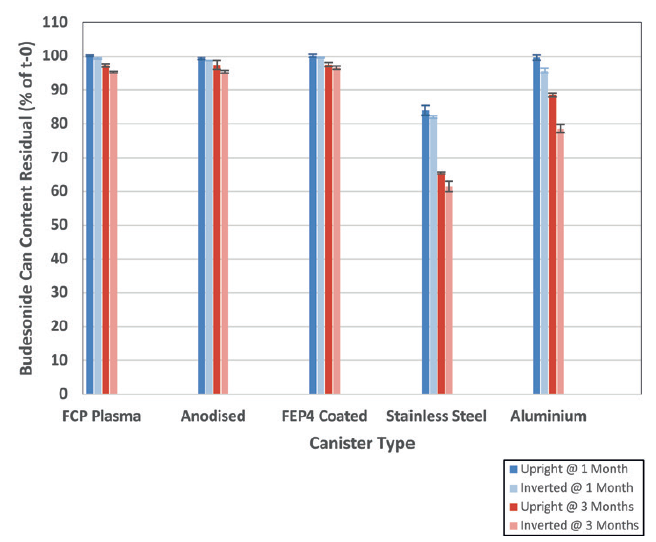
Figure 2: Percentage of residual budesonide content left in each can (relative to initial time point, t = 0) following storage at 40°C and 75% RH for 1 month and 3 months (mean ± standard deviation, n = 3).
Fluticasone Propionate Study – An Example
In a second study, aerodynamic particle size distributions (APSD) were evaluated for plasma, FEP and plain canisters with regard to a 125 μg fluticasone propionate suspension formulation (Figure 3).
The data show that at the initial time point, the plasma canister outperformed the FEP and plain canisters for APSD testing, showing improved performance and less adhesion with plasma canisters.
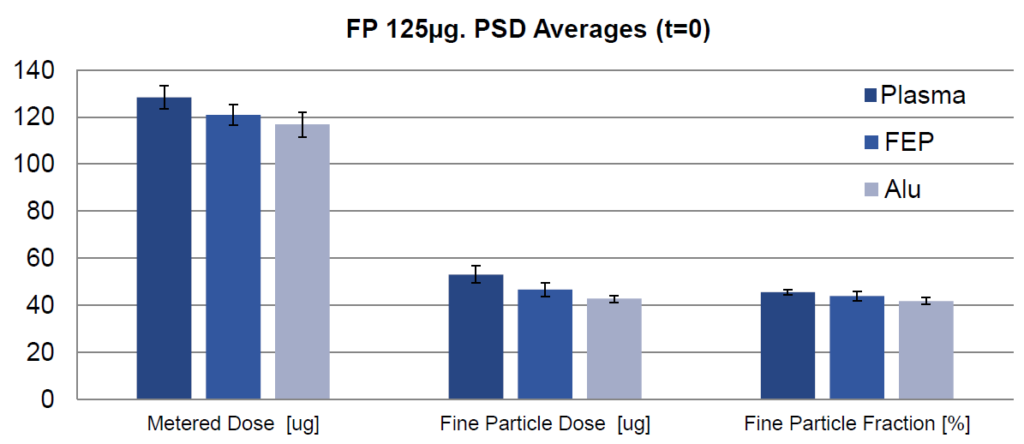
Figure 3: Data obtained at the initial time point for three canister types (mean ± standard deviation, n = 4).
“With the plasma treatment process being a singlestage, low-energy process, the energy consumption is 50% less than that for spray coating.”
SUSTAINABILITY
Plasma has the advantage of being able to form a nanolayer on the canister interior without the need for mass heating, due to the very high energies that can be confined thinly and consistently across all contours and high-aspect ratios of the canister. The lack of required heating means that a standard, thin-walled canister is able to be used, resulting in 30% less aluminium usage than traditional coating methods, which dictate the use of a thick-walled canister – and therefore increased costs.
With the plasma treatment process being a single-stage, low-energy process, the energy consumption is 50% less than that for spray coating. This reduced energy means that the carbon footprint of plasma canisters is significantly less than that of FEP-coated and anodised canisters (Table 1).
| Can Type | CO2 Per Million Cans (Kg) |
| Plasma | 143 |
| Anodised | 900 |
| Spray Coated | 1,445 |
Table 1: CO2 values for treated can types, per million cans. Data from H&T Presspart.
The lack of solvents used for plasma treatment means that there are no harmful emissions or measurable extractables or leachables. It is likely that the solvents and chemicals used for spray coating and anodising processes will fall under REACH “Registration, Evaluation, Authorisation and Restriction of Chemicals” (REACH) or similar regulations in the coming years, which will not be the case for plasma, ensuring these canisters are a future-proof solution.
CONCLUSIONS
The ever-increasing challenges with pMDIs arise from more complex and combination formulation developments, cost-reduction initiatives and the more recent pressure within the pharmaceutical industry around the need for improved sustainability.
Although propellant changes have the potential to improve the sustainability of pMDIs, there are also improvements that can be made by changing components. Analytical tests have demonstrated that plasma-treated canisters can provide improvements for both adhesion and degradation compared with plain, stainless steel, anodised and FEP-coated cans when used in conjunction with both budesonide solution and fluticasone suspension formulations.
Overall, the use of a Presspart plasma canister provides the most sustainable treated can option and tackles the two failure modes associated with pMDIs – improving pMDI performance and providing the most cost-effective, future-proof treated canister.
REFERENCES
- Vervaet C, Byron PR, “Drug–surfactant–propellant interactions in HFA-formulations”. Int J Pharm, 1999, Vol 186(1), pp 13–30.
- Baron C et al, “Evaluation of Powder Adhesion and Stability of pMDIs with Different Canister Types Including Plasma Treated Canisters”. Respiratory Drug Delivery, 2014, Vol 2, 2014, pp 349–354.
- Stevenson P, “Controlling Surface Performance Characteristics of Medical Devices: A Critical Review of the Latest Industrial Techniques”. Respiratory Drug Delivery, 2016, Vol 1, pp 147–156.
Previous article
SUSTAINABILITY, WASTE & REMANUFACTURING IN THE MEDICAL SECTORNext article
STRATEGIES TO ACHIEVE LONG-TERM SUSTAINABILITY
