To Issue 148
Citation: Neveu A, Lupo M, Francqui F, “Powder Characterisation for Pharmaceuticals: Beyond Standards.” ONdrugDelivery, Issue 148 (May/Jun 2023), pp 16–19.
Aurélien Neveu, Marco Lupo and Filip Francqui discuss the limitations of current standardised procedures for assessing the properties of pharmaceutical powders and how Granutools’ instruments – GranuPack and GranuHeap – offer a significant improvement over these existing standards, enabling keener insights and more repeatable analyses.
“It is essential to evaluate the powder properties precisely to optimise the formulations as well as develop materials with improved processability.”
INTRODUCTION
Powders are one of the most commonly used material forms in the pharmaceutical industry. In particular, powdered materials are core to the manufacturing process of pharmaceuticals for solid oral and dry powder inhaler (DPI) applications. The processes involving powders are usually constituted by different steps, such as the feeding of the material into the production line, the blending of several constituents (excipients, API, additives) and the actual manufacturing of the product (tablets, capsules or inhalation devices).
Each of these production stages is influenced by the properties of the powder. For example, the homogeneity of the blends will be reduced if working with strongly segregating powders. Furthermore, the flowability has a drastic impact on the consistency of the mass flow at the output of the feeders, which can lead to variability in the mix.
In applications such as the production of tablets, the powder has to fill a small die prior to the compression stage. This critical step has to be fully controlled, as it will directly influence the weight consistency of the final tablets. In particular, the rheology of the powder has been identified as an essential property to predict and control the mass variability in tableting processes.1
The properties of a powder can also play an important role during administration to the patient. Particularly with DPI applications, the small API particles have to reach targeted spots in the lungs. A common way to proceed is to attach the small API particles to the surface of a carrier made of larger particles. The API particles can then be carried through the throat and released into the lungs. The electrostatic properties of the particles play a fundamental role in this process.
Therefore, it is essential to evaluate the powder precisely properties to optimise the formulations as well as develop materials with improved processability. Powder properties can be evaluated in numerous different ways – a consequence of the lack of theoretical knowledge due to the complexity of the material. Contrary to classical fluids, for which the evaluation of the viscosity is usually sufficient to get a global picture of the flowing behaviour, there is no such universal metric for granular materials and powders up to now.
Over the decades, different methods for evaluating these material properties under specific solicitations have been developed. The most common ones consist of evaluating the angle of repose, the tapped density, the flowability and the response to shear. However, in these characterisation methods, the material is tested in a wide range of stress states, ranging from quasi-static to dynamic. Therefore, despite relying on the same micro-properties and interactions at the scale of the particles, each of these methods produces different information. Furthermore, a change in the characterisation protocol can lead to different results.
As an example, consider a very simple angle of repose (AOR) evaluation. A heap of powder is built and the angle of the heap that the powder naturally forms is measured. This angle depends on the properties of the particles, mainly their shape, and the particle–particle interactions, such as friction and cohesive forces. Measuring the AOR therefore gives an indirect evaluation of these properties. However, depending on the protocol that is followed to form the heap, different angles can be observed for the same powder. As a consequence, the results are influenced by the method protocol and the operator that performs the measurement. The necessity to define standardised procedures is thus unavoidable.
The most common characterisation methods used to evaluate pharmaceutical powder properties have been standardised by modern pharmacopeias and standardisation organisations. These standards are designed to provide well-established and easy-to-follow procedures. As everyone follows the same protocol, the results can be directly compared. These standardised procedures are especially useful when defining production criteria to guarantee that the material meets the expected quality. In fact, most of the regulations applied to pharmaceutical products rely on the evaluation of the powder properties according to standardised protocols.
However, the existing standards for powder property analysis are based on old and simple techniques, which can suffer from a lack of accuracy and repeatability. Moreover, the information gathered from these techniques is limited and is no longer sufficient for the current needs of high-end product development. This article focuses on two commonly used characterisation methods: tapped density analysis and the AOR.
TAPPED DENSITY ANALYSIS
Tapped density analysis is a very popular method for powder characterisation because of both the simplicity and the rapidity of the measurement. Evaluating the packing properties gives useful insights into the powder properties. Indeed, the way the particles rearrange during packing is influenced by the micro-properties of the material. In essence, the measurement is performed by first evaluating the apparent bulk density – the density obtained after pouring the powder into the measurement cell. Then, taps are applied to reach the tapped density corresponding to the maximum bulk density. A common metric extracted from this method is the Hausner ratio – the ratio of the tapped to the apparent density. The Hausner ratio is used as a flowability metric; the higher the Hausner ratio, the higher the cohesiveness of the powder and thus the lower its flowability.
Current Standards – Advantages and Drawbacks
The standardised tapped density protocol is fully defined in multiple pharmacopeias. A brief outline of the USP <616> protocol is discussed here. First, approximately 100 g of powder is poured inside a 250 mL graduated cylinder. Then, a predefined sequence of taps from a height of 3 mm is applied to pack the powder. The bulk density is evaluated after 10, 500 and 1,250 taps, and then followed by subsequent 1,250 taps sequences if the volume difference between two consecutive sequences is greater than 2 mL. For each bulk density evaluation, a naked-eye measurement of the powder volume is performed by the operator and, as the total mass is known, the bulk density is computed. Despite its long-term use, the tapped density standardised method still suffers from several limitations:
1. Initial powder bed levelling
After the pouring of the powder by the operator, USP <616> asks the operator to “carefully level the powder without compacting”. This powder levelling can induce variability in the apparent density measurement and may lead to unwanted initial packing of the material and an incorrect apparent density estimation. Moreover, the operator has a strong influence on this step, leading to operator-dependent results and reducing the advantages of using standardised procedures.

Figure 1: Measurement variability induced by visual reading in standardised tapped density protocols.
2. Visual assessment of the powder volume
The standardised tapped density measurement requires a visual evaluation of the powder volume. Therefore, the measurement accuracy will depend on the graduated cylinder precision (2 mL) but also on the operator performing the volume reading. Indeed, for moderate to highly cohesive powders, the powder-air interface is very irregular (Figure 1) and the operator will usually determine an average interface position to estimate the volume of powder. Moreover, if the graduated cylinder is not perfectly levelled during taps, a slope can appear in the powder bed. In this case, the measurement accuracy and operator dependency are even worse.
3. Large amount of powder required
The standardised measurement requires a large amount of powder – approximately 100 g. However, this amount of powder may not be available due to production or cost constraints, especially in the formulation development process.
Improved Method – GranuPack
Due to the lack of accuracy inherent in the standardised method, the insights gathered from this measurement can have limited applicability – it is difficult to highlight small differences in properties. In particular, when performing batch-to-batch evaluation to detect drifts in production, small changes in powder properties have to be evidenced, as they can lead to large variations in final product quality.
To tackle these limitations, an improved tapped density instrument has been developed by GranuTools based on the most recent fundamental research.2 The GranuPack provides high accuracy and guarantees low operator dependency. With the GranuPack, only 35 mL of powder is required to perform a tapped density measurement.
To obviate user dependency on the initial pouring, a special initialisation protocol has been designed for the GranuPack. First, an initialisation tube is placed into the measurement cell (Figure 2), into which the operator pours the powder. Then, the tube is automatically moved upwards to pour the sample into the measurement cell in a user-independent and repeatable way. A light, diabolo-shaped piece is placed on top of the powder to ensure a flat powder surface. Finally, taps are applied and a sensor precisely measures the height of the powder after each tap.
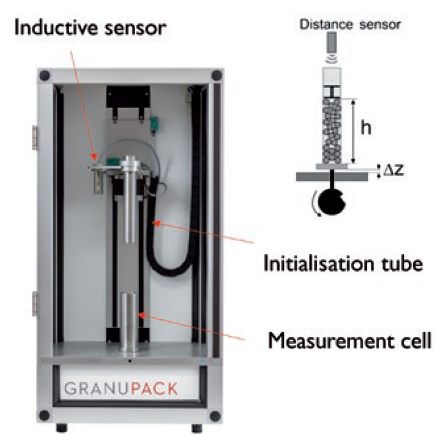
Figure 2: GranuPack – an improved tapped density analyser.
“The AOR protocol has been improved upon by the GranuHeap instrument – an automated heap shape measurement method based on image processing and analysis.”
The GranuPack assesses the complete packing curve, enabling a level of investigation into the packing dynamics that is not possible with the standard protocol. This allows for gathering useful new insights into the powder properties. Figure 3 shows a comparison between the procedure laid out in USP <616> and the GranuPack protocols for two lactose powders. In both cases, the obtained Hausner ratio is similar, but the repeatability is drastically improved with the GranuPack protocol.
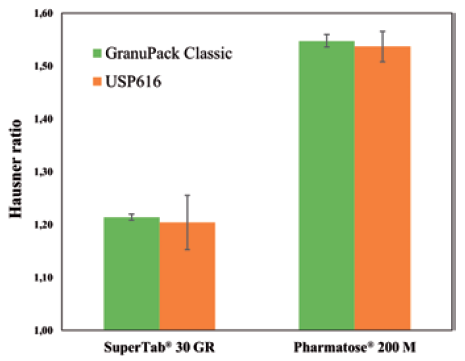
Figure 3: Hausner ratio measured with the GranuPack Classic and the USP <616> procedure for both powders. Error bars are ± the standard deviation on three independent tests.
ANGLE OF REPOSE
Another commonly used powder characterisation method is measuring the AOR. The AOR directly depends on the internal friction of the material and the strength of its cohesive forces. The higher the AOR, the higher the cohesiveness and thus the lower the flowability. Unfortunately, the protocols defined in USP <1174> and EP 2.9.36 do not give a strict procedure to measure this angle, which can induce operator-to-operator variability. Therefore, the standardised AOR evaluation does not provide the precision required to perform a fine evaluation of drifts in powder properties.
The AOR protocol has been improved upon by the GranuHeap instrument2 – an automated heap shape measurement method based on image processing and analysis (Figure 4). The operator poursthe powder into an initialisation tube, which then moves upward at a constant speed of 5 mm/s, allowing the powder to form a heap on a cylindrical support in a repeatable way. Then, the base slowly rotates and pictures of the heap at different angles are taken and analysed by a custom image recognition algorithm to determine the position of the powder-air interface and compute the AOR.

Figure 4: GranuHeap – an automated AOR measurement device.
This protocol removes the user dependency of the AOR evaluation, which is particularly important when evaluating cohesive powders. Therefore, the GranuHeap provides an accurate and repeatable way to measure the AOR and constitutes a major improvement over the standardised procedure.
CONCLUSION
Defining standardised characterisation protocols is essential to guarantee the quality of the products and to ensure that they meet the strict regulation criteria. However, the characterisation protocols on which the current standards rely are based on old and overly simple methodologies that do not allow for fine evaluation of the material properties. With the constant development of high-end pharmaceutical products and processes, such as the transition from batch-to-batch to continuous manufacturing production, there is an increasing need for more refined characterisation methods.
Fortunately, new and improved powder characterisation tools have been developed during the last decade. In particular, the GranuPack and GranuHeap devices presented in this article provide a major improvement to tackle the limitations of the standardised protocols. The industry must now move forwards and upgrade the current standards to use these high-precision, state-of-the-art powder characterisation methods.
REFERENCES
- Janssen PHM et al, “Impact of Powder Properties on the Rheological Behavior of Excipients”. Pharmaceutics, 2021, Vol 13(8), article 1198.
- Lumay G et al, “Measuring the flowing properties of powders and grains”. Powder Technol, 2012, Vol 224, pp 19–27.


