Citation: Duband S, “Tackling High-Volume Administration Challenges with a Smart, Sustainable On-Body Injector Platform”. ONdrugDelivery, Issue 124 (Sep 2021), pp 36–40.
Séverine Duband introduces Symbioze, Nemera’s novel on-body injector platform device, comprising a reusable main unit and disposable prefilled drug container, and offering a balance between the need to deliver advanced formulations at high volumes and the desire for patient-centric ease-of-use that enables patients to self-administer their therapies at home.
Over the last decade – driven by significant innovations in APIs, drug compounds, formulation technologies and manufacturing processes – drug administration has become increasingly complex, with significant changes in pharmacokinetic profiles (viscosity, volumes, physiological properties, stability, etc), and, based on the current outlook, this is an accelerating trend. This rising complexity has generated several challenges from both the perspective of both the drug and the device side.
In parallel, major market trends have emerged,1 such as:
- Rising prevalence of chronic diseases
- Shift towards at-home care
- Growth in the biologics market
- Connected health technologies
- Shift towards value-based care.
All of which are opening the pathway for a new generation of injectable devices (Figure 1).
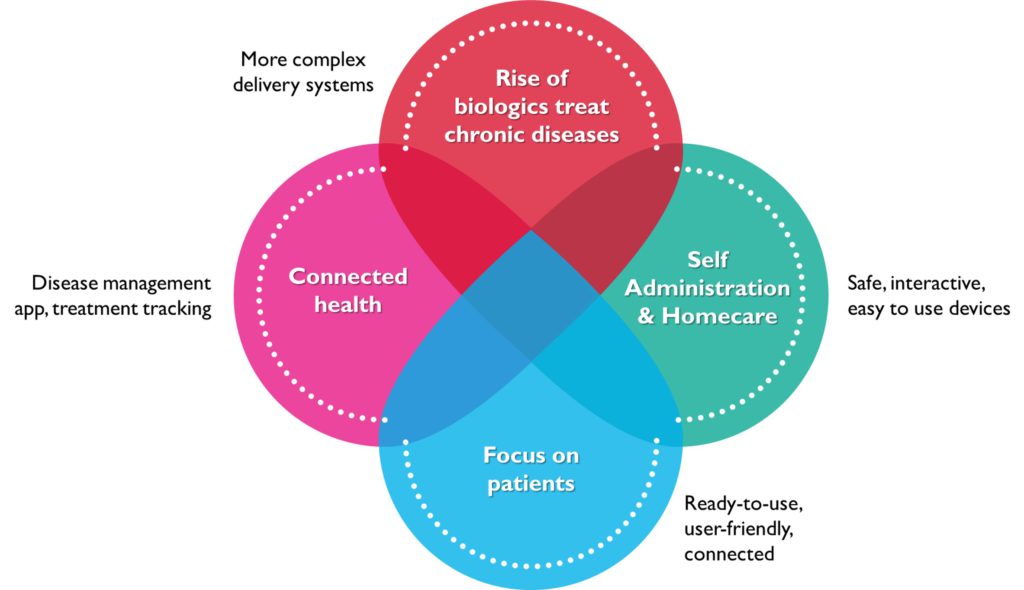
Figure 1: Major market trends impacting the parenteral space.
CHALLENGES FACING MODERN DRUG DELIVERY DEVICES
Within this continually evolving environment, a recurring issue that generates a need for innovation in drug delivery systems is the increasing volume of drug required for each administration. Whereas the gold standard for self-administration used to be the 1–3 mL range, more and more treatments now require volumes of over 5 mL per injection, with some even requiring volumes as high as 20 mL. This is driven mainly by:
- The high molecular mass of certain drugs, especially monoclonal antibodies (mAbs).2
- Manufacturing challenges on fill-finish lines, leading to the need to dilute the formulation, thus increasing its volume
- Increased concentration of formulations in order to decrease the required frequency of administration.3
- New treatments requiring high dose volume linked to patients’ body weight, such as immunoglobulins.
Another substantial advance in the injectable space is the ongoing shift from intravenous (IV) to subcutaneous (SC) delivery routes.3,4 This move from IV to SC brings many benefits, including:
- Reducing time spent in hospital, or avoiding the need for a healthcare professional altogether, which relieves patients from the burden of frequent visits to clinics and decreases cost for payers.
- Decreasing the risk of adverse events, such as systemic infections, blood clots and air embolisms.
- Allowing a less-invasive procedure, increasing patient acceptance of and adherence to their treatment.
- Offering the option of self-administration, which gives greater control to patients in managing their therapies.
“Within this continually evolving environment, a recurring issue that generates a need for innovation in drug delivery systems is the increasing volume of drug required for each administration.”
This shift has been further accelerated by the covid-19 pandemic, with a strong push for medications that can be administered at home to avoid unnecessary exposure to the virus at healthcare facilities and to free up medical care resources. With a growing number of patients seeking independence and convenience in managing their treatment, the push for self-administration is inevitably growing stronger.
Finally, the frequent use of self-injection devices, combined with the adoption of “smart” connected devices, is adding a further layer of complexity and increasingly raising concerns around their environmental impact. With the majority of marketed connected devices today being fully disposable, electronic waste management and device disposal are creating safety and sustainability issues for the industry.
Therefore, the question that presents itself is how best to answer these challenges while still providing patients with the best possible care? Is there a solution capable of accommodating a high-volume SC injection with a seamless, sustainable and successful self-injection device?
DELIVERING LARGE VOLUMES AT HOME IN A SUSTAINABLE AND USER-FRIENDLY WAY
Even with the variety of injectable devices already developed and commercialised, it is no easy task to select a suitable solution for the delivery of complex, large-volume drugs that also enables patient self-administration, or at least at-home administration. Whilst there are several easy and ready-to-use offers available for patients to choose from to self-administer their medication,4 these are very often limited in the drug volume that can be injected:
- The largest standard size of prefilled syringes (PFS) is limited to 2.25 mL
- Injection pen devices can usually only deliver drugs in cartridges up to 3 mL
- Autoinjectors are usually based on either a cartridge (1–3 mL) or a PFS (1–2.25 mL).
Therefore, if the drug volume needs to be above 3 mL, then the only available option is an on-body delivery system (OBDS). However, OBDSs usually involve a pump-based administration, which comes with its own set of challenges, mostly user- and environment-related (Figure 2).
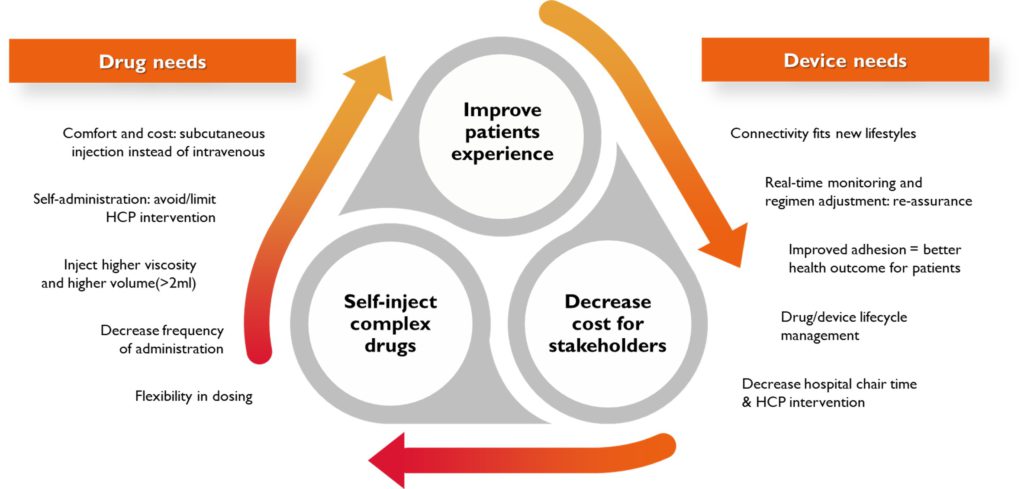
Figure 2: OBDSs are often the only option to tackle drug administration and device delivery constraints.
Traditional pump-based systems are complex devices and often come with a difficult set-up process involving a drug transfer operation, lots of tubing and requiring a sterile setting. As such, their use often requires the involvement of a healthcare professional or ends up being a real burden if the patient attempts to regularly self-administer.
More recently, a new generation of patch-pump devices has been launched, demonstrating significant progress in miniaturising components and making the use steps easier. These devices help to enable deported devices to be self-administered at home. However, these solutions still suffer from some limitations:
- Drug volumes are often limited to 5–10 mL
- Device set-up frequently involves a drug transfer operation or inserting a prefilled primary drug container into the device
- Electro-mechanical components (pump engine, sensors, connectivity) are embedded in the device and must be disposed as hazardous waste, which is neither cost-effective nor sustainable.
“The Symbioze smart wearable platform is Nemera’s latest development in injectable devices, designed to reconcile the injection of complex drugs with stakeholders’ most demanding needs.”
In short, even current wearable device options are creating hurdles for both patients (risk of use errors with drug container manipulation, complexity limiting adherence and therefore treatment efficacy) and for pharmaceutical companies (complex manufacturing processes, poor sustainability footprint), which could limit the adoption of such devices. Nemera has been striving to find ways to answer these needs, and has recently developed an innovative solution to reconcile the need for large-volume injections with the desire for self-administration in a user-friendly and sustainable platform.
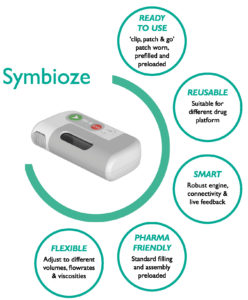
Figure 3: Symbioze – Nemera’s on-body injector platform.
NEMERA’S SYMBIOZE ON-BODY DELIVERY SYSTEM
A Differentiated Approach in the Wearables’ Landscape
The Symbioze smart wearable platform (Figure 3) is Nemera’s latest development in injectable devices, designed to reconcile the injection of complex drugs with stakeholders’ (patients, healthcare provider and payers) most demanding needs. Combining a robust design with ease-of-use, connectivity and sustainability, Symbioze fosters patient adherence by way of a seamless and enhanced injection experience. This innovation is specifically tailored for the administration of complex drugs, such as biologics, by means of a highly engineered, reliable drug delivery system. It is suitable for very large volumes, up to 20 mL and beyond, while preserving formulation integrity – critical for mAbs.
Another major differentiator for Symbioze in today’s landscape is the choice to go with a disposable unit and a reusable system housing the main drive module (Figure 4). The benefits of a reusable part are two-fold:
- Sustainability: OBDSs usually involve electro-mechanical components, for both injection control and connectivity purposes. Embedding those into a reusable unit offers a more favourable environmental footprint, especially with regard to the device’s waste management profile.
- Cost efficiency: As it is designed to be used multiple times, using Symbioze is a profitable choice, especially in a value-based care environment that often involves expensive therapies. This is even more beneficial when targeting medications requiring large administration volumes.

Figure 4: Symbioze consists of a reusable main unit and a prefilled, preloaded disposable module containing the drug.
The drug product is contained in the disposable part, with the drug container already prefilled and preloaded for patient safety and ready-to-use convenience. An embedded recognition system ensures that drug information verification is shared between the reusable and disposable elements.
As the target of Nemera’s OBDS is to be able to accommodate a wide range of applications, adopting a platform approach was a must. Symbioze is designed with enough flexibility to be adjusted to meet the needs of any pathology, targeted patient population and drug posology. This also enables multiple uses of the device with a portfolio of therapies, leveraging its reusable benefit for pharmaceutical players.
Symbioze offers a unique drug delivery system including following benefits:
- Adaptable to several drug volumes and viscosities
- Adjustable injection speed
- Injection control and failure mode control
- Fully integrated engine in reusable core system.
At the Crossroads of Industry and Patient Needs
From the start, the Symbioze OBDS was developed with the goal of finding the best possible compromise between meeting pharmaceutical industry standards and patient needs. OBDSs are both highly complex devices and still novel to the healthcare industry, with only a few drug/device combination products registered and marketed. Therefore, it is critical to stay within the industry manufacturing standards, in respect of both processes and components, to decrease global risk profile and limit investment requirements. Some of Symbioze’s features include:
- A standard primary drug container (PDC), a glass cartridge, in a nest-and-tub configuration. This allows the use of standard filling equipment, either by the customer or a contract filler.
- An innovative, patent-protected design allowing prefilled PDC assembly in the disposable unit in a non-aseptic environment. An embedded sterile connection between the drug container and the fluid path enables both a ready-to-use solution for patients and a seamless filling and assembly process for manufacturers.
Finally, Nemera needed to ensure that, throughout the development, no compromise was made on the injection experience from a patient’s perspective. The company leveraged its Insight Innovation Center expertise to map out the envisioned patient journey, which is an even more critical process when considering new, complex devices. This process includes the development of a human factors task analysis, which identifies the demands placed on the user, assuming the general workflow and system components, and notes potential use errors. This effort sets the foundation for the human factors programme for potential applications.
Integrated task analysis allows for the mapping of key user needs at each step of the workflow, identifying potential challenges or errors, and for appropriate prioritisation of development activities. This allowed Nemera to set a foundation for Symbioze to ensure that the device is optimised across all aspects of device use from set up and wear to removal and storage in a home-use context (Figure 5).
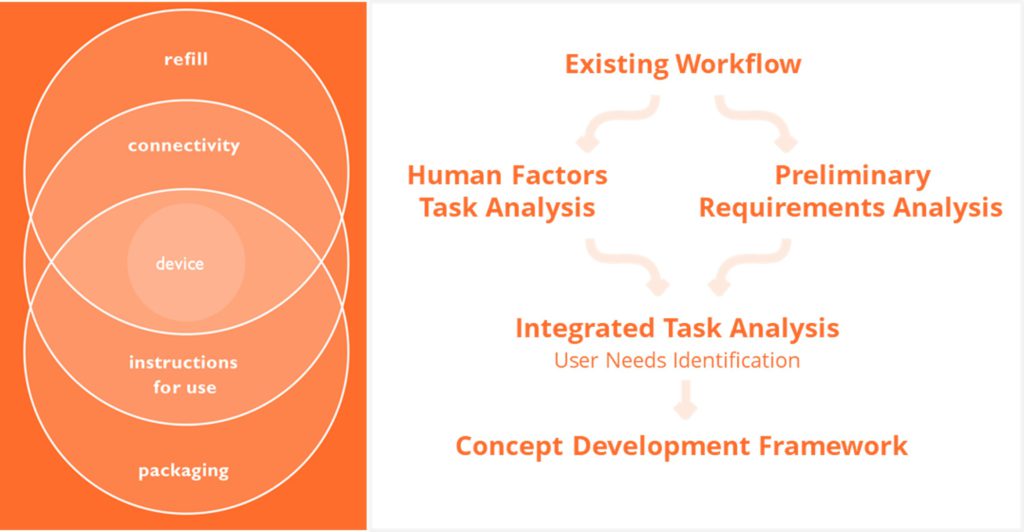
Figure 5: Supporting the combination product ecosystem from the earliest stages of developing a device strategy.
This effort was translated by Nemera’s team of experts into a concept development framework (CDF) that outlined a list of user needs that needed to be met by the design of the device and provided a clear priority order of tasks for achieving that aim, based on acquired knowledge. The CDF was used to guide the development process and ensure alignment with a hierarchy of user needs related to technical challenges. This methodology drove development of a platform that is as broadly applicable to potential patient populations and drug product characteristics as possible. This foundation will be supported by multiple studies that are planned to take place over the course of the development of Symbioze. A key aspect to assess at the current stage of development is how the combination of size, weight and adhesive for a 20 mL device impacts on “wearability” during use, given a wide variety of potential wearing times.
By applying a thorough understanding of the patient journey and the broader healthcare ecosystem to the development of Nemera’s device strategy for Symbioze, the company has established a foundation that can be applied to its customers’ specific applications. Nemera can work with customers to adapt Symbioze to their specific needs, as well as provide services to support their combination product development requirements – including differentiation of the user experience and support of clinical trials from a device supply perspective, as well as the rest of Nemera’s broader capabilities.
The Right Balance Between Drug Delivery
Technology and Patient-Centricity The right balance between simplicity and robustness of an OBDS is crucial for ensuring seamless self-administration, without adding unnecessary complexity and thereby compromising the patient experience. Symbioze offers sustainability, provides extra protection to users and ease-of-use, which are some of the most important characteristics in modern advanced parenteral devices. Nemera’s extensive end-to-end capabilities in design, development and manufacture, coupled with Symbioze’s platform approach, embraces the need for specific combination product solutions tailored to unique patient populations.
REFERENCES
- Ouensanga A, “Drug Delivery Devices (DDD): Increasing Role in Patient Outcome and Future Trends, New England’s Global Appeal to Foster Collaboration”. Alira Health, Mar 2021.
- Johnson B, Rostovtsev A, “High Concentration Biologic Formulations: Challenges and Solutions”. Drug Discovery & Development, Jun 2017.
- Badkar AV et al, “Subcutaneous Delivery of High-Dose/Volume Biologics: Current Status and Prospect for Future Advancements”. Drug Des Devel Ther, Jan 2021, Vol 15, pp 159–170.
- Bittner B, Richter W, Schmidt J, “Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities”. BioDrugs, Oct 2018, Vol 32(5), pp 425–440.

