Citation: Penman M, “Expert View: The Challenges of Verifying a Wearable Infusor”. ONdrugDelivery Magazine, Issue 100 (Sep 2019), pp 38-41.
Michael Penman shows why it is important to verify body-worn infusor performance, highlighting the challenges and learnings from an example where Team Consulting was tasked with the design and verification of one such wearable device.
A wearable infusor can make a dramatic difference to patients’ lives but to ensure that it is effective, it must be tested for performance and safe operation in all foreseeable environments and interactions.
“Design verification at
the system level aims to
assess the wearable infusor’s performance and safety against all foreseeable environments and interactions.”
Foreseeable Environments
Patients using the wearable infusor we tested were unlikely to partake in strenuous activities. With that in mind, the design aimed to avoid adding any further limitations to the daily life of such patients. This meant the wearable infusor had to be tested in all environments it might foreseeably be worn. From a cool and dry winter’s day in Beijing, to a hot and humid summer’s day in Hong Kong; from the high atmospheric pressure at the shores of the Dead Sea, to the low-pressure heights of La Rinconada, Peru; and so on. These examples may sound extreme, but some of these environmental changes exist in day-to-day life for most of us: from sitting in a cool and dry air-conditioned office, to having a hot and humid shower; pressure changes in the aircraft cabin during a flight, and so on.
Foreseeable Interactions
There are several predictable interactions a continuous-delivery wearable infusor may encounter whilst worn by a patient carrying on with their day-to-day routines. These range from being knocked on a piece of furniture, crushed by a portion of the patient’s weight, shaken on a bumpy road, experiencing changes in back pressure when a patient transitions from lying down to standing up, etc.
SYSTEM LEVEL APPROACH
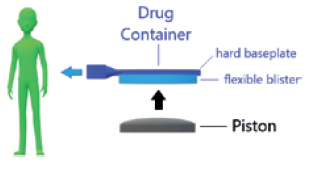
Figure 1: Drug delivery mechanism.
Design verification at the system level aims to assess the wearable infusor’s performance and safety against all foreseeable environments and interactions.
Delivery Accuracy Performance
The wearable infusor was designed to deliver drug at a flow rate of 42 μL/hour with a nominal tolerance of ±6% on the total dose. Delivery is achieved by using electronics and a bespoke expanding component to displace a piston, which in turn pushes the flexible blister of a drug container (Figure 1). The rate of displacement of the piston is kept in check by a proportional derivative control system. To achieve the intended rate of delivery, the target rate of piston displacement isset to 34 μm/hour. Put in context, the pace at which the piston moves is only slightly faster than the rate at which hair grows from the human scalp.1
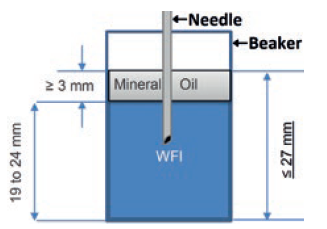
Figure 2: How the collection beaker is set up to capture mass changes used to calculate delivery accuracy.
In design verification, delivery accuracy was determined by continuous logging of the mass change on a precision balance, weighing a collection beaker set up as shown in Figure 2. Note the layer of oil, needed to avoid evaporation which if allowed to occur would reduce the measured change in mass, and thus the perceived delivery accuracy by approximately 30%.
Delivery accuracy had to be assessed for a set of wide-ranging circumstances. This meant the set-up described had to be possible in all the test environments shown in Figure 3. A lot of method evaluation was necessary to optimise the test protocols for each scenario.
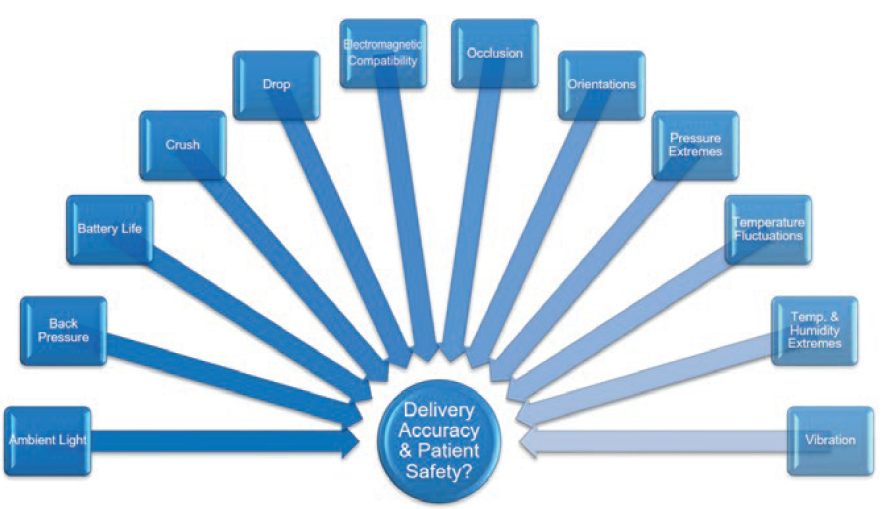
Figure 3: System design verification tests assess delivery accuracy and patient safety during delivery.
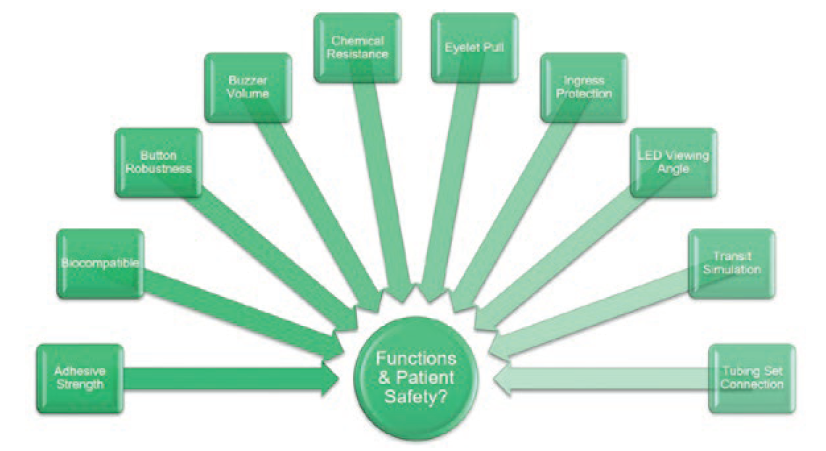
Figure 4: System design verification tests assess additional performance requirements and patient safety.
Additional Performance Requirements
In addition to tests assessing delivery accuracy, a separate suite of tests was conducted, assessing design features with their own performance requirements (Figure 4). For example, audible and visual indicators were required to be observable from 1 m; the skin adhesive patch had a minimum peel force; the user status button had to be robust; the enclosure had to be protected against ingress by water and solid foreign objects; etc.
CHALLENGES AND LEARNINGS
Bubbles
Initial testing revealed some periods of unexpectedly erratic delivery accuracy. Investigations into these disturbances concluded that the root cause was the presence of air bubbles in the drug container of some of the wearable infusors. The transfer of these bubbles to the collection beaker was observed to cause the erratic measurements.
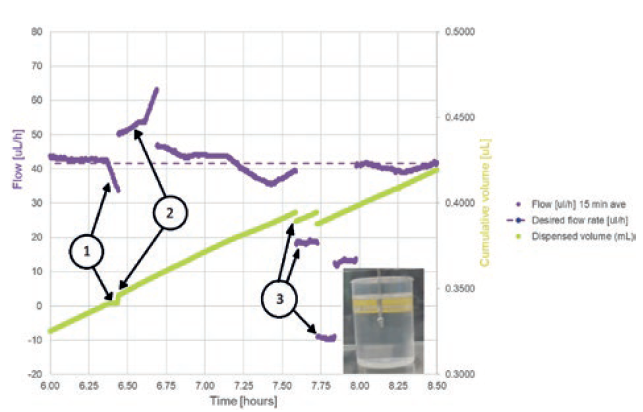
Figure 5: Test artefacts that are introduced by air bubbles.
Preliminary test data (Figure 5) shows three effects on the logged mass (converted to volume in the figure) and the calculated flow rate:
- A temporary occlusion, causing cumulative volume figures to stay constant and 15-minute-averaged flow to start to drop. This was caused by an air bubble reaching a constriction.
- A short-term rise in flow rate, caused
- by the air bubble passing the constriction and the air having a viscosity around 50 times lower than fluid, allowing it to move through the wearable infusor’s fine bore tubing set more quickly.
- Step decreases in flow rate and the drops in cumulative volume, caused by bubble-release in the collection beaker. Bubbles were captured exiting the needle in the collection beaker at these points, confirming the theory.
The Effects of Accelerated Ageing
Accelerated ageing is a useful tool for testing the performance of a system at the end of its shelf life sooner than would otherwise be possible. The effect of elevating temperature to speed up the ageing process of most materials is reasonably well understood, so much so that ASTM International (formerly American Society for Testing and Materials) has a Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices.2 However, the overall effect on a complex system will contain unknowns.
Preliminary test runs on accelerated aged wearable infusors showed side effects, leading to design changes. The initial design of the wearable infusor included a battery voltage check within the first few milliseconds of start-up. Devices tested after accelerated ageing failed this check, rendering them unusable. Investigations showed that the batteries were not in fact defective and returned to acceptable start voltages within seconds of start-up (Figure 6). The low initial voltage was due to passivation, a film of lithium chloride that forms on the surface of the lithium anode giving protection against excessive self-discharge. It led to a more sophisticated battery voltage start-up check taking measurements over the first 40 seconds of operation. A simple fix, but a good reminder that a preliminary round of testing, including any ageing intended to be part of the formal test programme, is essential to avoid unwanted delays. Furthermore, it is always advisable to start a real-time ageing programme in parallel in order to differentiate between the representative and unrepresentative effects of accelerated ageing.
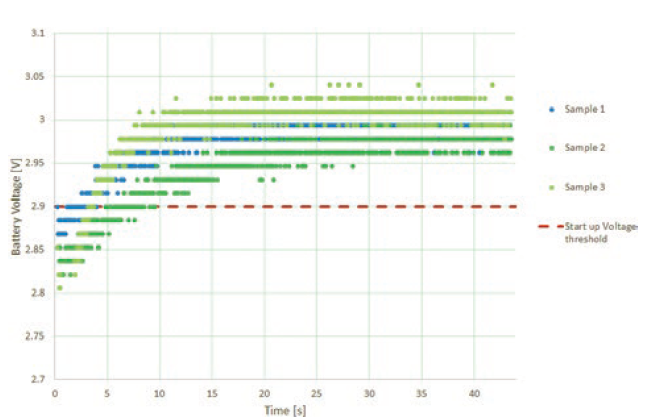
Figure 6: Battery de-passivation can occur after ageing.
Data Capture
Paper forms were used to capture test operator actions, observation, etc. in the preliminary test programme. Efforts were made to minimise the number of custom forms needed. This meant there was a lot of cross-referencing, and keeping all the forms relating to the tests performed on a device was a significant administrative burden.
This led to the development of database-driven electronic data capture forms, allowing a built-in audit trail and report generation. This simplified the data capture and reduced the administration needed. The electronic data capture form databases themselves had to be validated, which was no mean task, but once that was done, operators, reviewers and report writers found themselves in a much better position to work efficiently.
SUMMARY
It takes a lot to design and verify a medical device that will keep patients safe whilst providing them with a therapy that improves their condition. We’ve not touched on the lower level verification programmes needed for the wearable infusor’s electronics, software or critical components in this article – rest assured each of these were equally thorough and challenging. Add the user studies needed to ensure the device is useable and will have little to no negative impact on patients’ daily activities, and you have a substantial task on your hands.
The EN 60601 Medical Electrical Equipment and Systems family of standards provided by the British Standards Institute (BSI Group) is an excellent place to look for test methods to ensure a medical device like the wearable infusor will perform and be safe. Ignore them at your peril. However, they must be carefully interpreted and customised for the unique requirements of each device.
REFERENCES
- Loussouarn G, Lozano I, Panhard S, Collaudin C, El Rawadi C, Genain G, “Diversity in human hair growth, diameter, colour and shape. An in vivo study on young adults from 24 different ethnic groups observed in the five continents”. Eur J Dermatol, 2016, Vol. 26(2), pp 147–148.
- ASTM F1980-16, “Standard Guide for Accelerated Aging of Sterile Barrier Systems for Medical Devices”. ASTM International,West Conshohocken, PA, 2016, www.astm.org

