Citation: Morgan V, “How Large-Volume Wearables are Revolutionising the Patient Experience”. ONdrugDelivery Magazine, Issue 100 (Sep 2019), pp 54-57
Victoria Morgan looks at how the combination of large-volume medicines and the trend for wearable devices is transforming life for patients, highlighting the company’s new-generation SmartDose 10 mL platform.
In recent times, various media outlets have promoted the comeback of flared trousers but, whilst they have been many a glam rock fan’s chosen attire for years, the mainstream fashion-adhering public is yet to be convinced. The 1970s was the only decade to fully embrace flares with open arms until the trend was pushed aside for the skintight, drainpipe jeans of the 1980s. Today, the skinny jean (a young relative of the drainpipe jean), with the help of a generous dose of spandex, seems to have stood the test of time.
“Designing a cost-effective drug administration process that offers a better experience for the patient is a radical change that will propel large-volume injection into a new era.”
Trends are constantly influencing our way of life and preferences. The same is true for healthcare, and patient preference has never been more firmly rooted in pharmaceutical development. From implants revolutionising the choice of contraceptive dosing to pens for top-up insulin injections at the dinner table, significant steps forward have been made in health management, thanks to patient preference. Such steps have been supported by pharma companies to help improve patient compliance and stay ahead of the competition.
Large-volume medicines have traditionally been infused or administered intravenously due to the challenges of getting the required volume of drug into a patient’s bloodstream. Patient self-administration of large volumes using traditional devices such as autoinjectors, pens or prefilled syringes has been unsuccessful for several reasons, such as the difficulty for a patient to hold a device in place for the required amount of time, the high viscosity of the drugs to be administered and the inability of subcutaneous tissues to absorb large drug volumes.
These challenges usually required patients to travel to health clinics and hospitals for treatment. Whilst the patient experience when visiting a hospital may vary, the interruptions to daily life, cost of travel, and the constant reminder of their disease state have driven the need for pharma manufacturers to consider the overall patient experience when developing alternative administration options.
While the patient always comes first, the cost of caregiving must also be considered during drug development. Designing a cost-effective drug administration process that offers a better experience for the patient is a radical change that will propel large-volume injection into a new era.
“The increase in combination products means more opportunities for device innovation for pharma companies, patients and payers.”
The invention of wearable devices and the availability of Enhanze® drug delivery technology from Halozyme (San Diego, CA, US) have been upstream changes that have met with the downstream desire of patients to have more influence over their disease management. These two technology advancements have allowed larger volumes to be administered subcutaneously, over a longer period, in a non-clinical setting by ensuring user requirements are at the heart of the patient experience.
This step change in drug packaging has been most applicable for biologic drug administration where viscous formulations and higher volumes are common. The new biologics pipeline continues to grow, with a focus on lifecycle management and total cost reduction. The number of preclinical and Phase I/II pipeline products increased 70% and 32%, respectively, from 2016 to 2018.1
Pipeline biologic molecules are focused on narrowly targeted therapies and small patient populations with reduced side effects and reduced dosing. The current trend is for consistent growth in combination products coming to market with a CAGR between 2014 and 2018 of 12%.2 Subcutaneous injections have rapidly expanded beyond diabetes and autoimmune therapy areas into cancer, cardiology and blood diseases, amongst others (Figure 1).
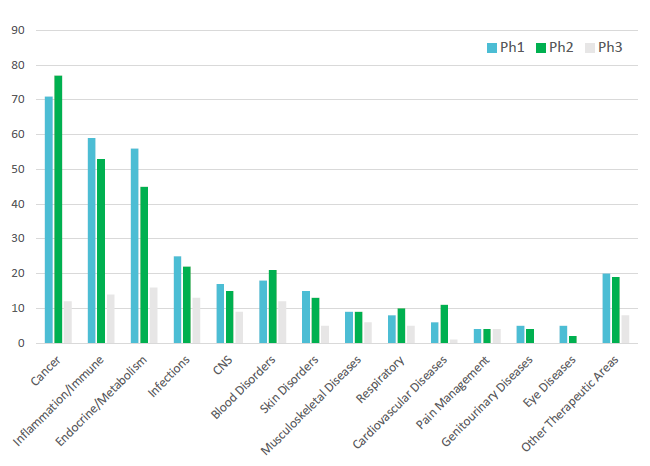
Figure 1: Number of new molecular entity subcutaneous biologics programmes in the clinic.3
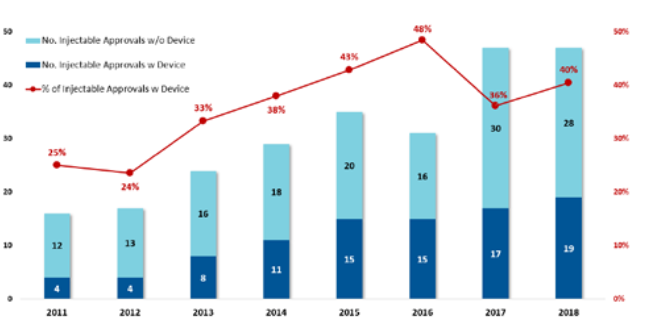
Figure 2: The increase in combination products means more opportunities for device innovation.
Not only are the number of new molecular entities in development increasing, but we also see drug development companies looking to reformulate existing commercial molecules into volumes suitable for wearable injectors. These companies are increasingly comfortable with bringing combination products to market (Figure 2).3
This sustained strong growth in the number of combination products means more opportunities for device innovation for pharma companies, patients and payers as follows:
-
- For the pharma company, drug delivery methods can be used to protect market share from biosimilar competition (such as with Amgen’s Neulasta® Onpro®)
- For the patient, higher-volume chronic disease subcutaneous injections offer advantages with less frequent injections, improved adherence and a home setting
- For the clinic/payer, a move from intravenous to subcutaneous drug delivery offers cost savings since:
- Faster drug prep time improves pharmacy efficiency4
- Fixed dosing reduces drug waste and medication error5
- Less set-up reduces nurses time.4

Figure 3: Amgen’s Pushtronex® system that incorporates West’s SmartDose® drug delivery technology.
West Pharmaceutical Services recognised these trends over a decade ago and invested in wearable technology in 2010. West’s first offering was the wearable and programmable SmartDose® drug delivery device, the technology of which Amgen incorporated into its Pushtronex® device (Figure 3). In 2016, the US FDA approved the use of Amgen’s Repatha® for hyperlipidaemia in the Pushtronex device, which was the first FDA-approved commercial-use wearable. Human factors showed the design, functionality, size and comfort were all conducive to the patient whilst allowing Amgen to differentiate its offering to the market.
West’s SmartDose wearable first-generation device revealed the need for higher-volume, subcutaneous delivery with programmable features to suit the dosing regime of the therapy. As a result, West expanded its commitment to wearable technologies with the development of a platform of devices. In January 2019, scPharmaceuticals (Burlington, MA, US)announced a development agreement with West for its Next-Generation FUROSCIX® On-Body Infusor, featuring second-generation technology of the SmartDose device.

Figure 4: Second-generation SmartDose® 10 mL wearable device.
FUROSCIX is a proprietary furosemide solution formulated to a neutral pH to allow for subcutaneous infusion via a wearable, pre-programed drug delivery system that is applied to the abdomen for subcutaneous drug administration. FUROSCIX is being developed for treatment of oedema, or fluid overload, in patients with heart failure. FUROSCIX has the potential to provide an outpatient alternative for the treatment of worsening heart failure due to oedema.
With considerable market traction around the SmartDose drug delivery platform, it is not surprising to see the results from West’s early recognition of the trend for larger volume delivery. The new-generation platform (Figure 4) offers usability and feature enhancements, including:
- Up to 10 mL delivery
- Better usability
- Faster Injection
- Quieter
- Training system (Figure 5)
- Filling pathway.

Figure 5: Training pack for onboarding caregivers and patients.
These usability and feature enhancements have been examined in extensive human factors testing. The extensive design incorporated body mass index (BMI), age, health status and experience and was arranged to test design usability, acceptability, comfort, whether the device addressed the patient needs and what was the monthly preference for administration. Study users chose the SmartDose 10 mL as an acceptable treatment, and rated it higher than all of the alternatives (Figure 6), which included autoinjectors, visiting a clinic for intravenous injection or infusion and even multiple doses with the lower-volume first-generation SmartDose device.
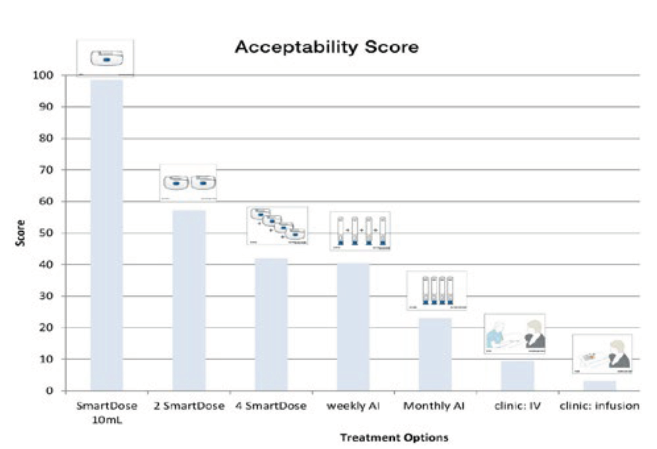
Figure 6: Human factors study results showing SmartDose® 10 mL was the preferred device.
FILL-FINISH IS A CRITICAL BRIDGE FROM DEVELOPMENT TO DELIVERY
Fill and finish services for wearable containers are a complex yet critical part of the drug development supply chain. Requirements for fill and finish services range from small-scale fills suitable for taking to the clinic, through to commercial-scale production volumes. Finding a partner who can support a drug developer’s requirements in the right way, at the right time, without risk to the overall development timelines can be time consuming.
Recognising the need to strengthen the device offering and taking more of a collaborative, integrated approach to supporting a customer has been a key driver for West. The company offers in-house small-scale laboratory filling services to help customers from sample preparation for product testing through to product and process characterisation. West can support a drug development company with GMP filling at established contract manufacturing organisations or enable a customer’s new fill line or retrofits in-house.
Earlier this year, West announced that it had commenced discussions with Swissfillon (Visp, Switzerland) – a provider of aseptic fill and finish services to pharma and biotechnology companies, that are intended to lead to a non-exclusive global collaboration to provide fill-finish capabilities to customers using the SmartDose® platform for complex molecules.
Through the collaboration, it is anticipated that West will be able to deliver an integrated solution with filled Crystal Zenith® cartridges for the SmartDose drug delivery platform, which is expected to accelerate clinical development and enable customers to bring their innovative injectable drugs to market quickly. This new collaboration is expected to offer customers a robust clinical fill-finish capability later this year.
West has seen many changes over the past decade from building out its device platform, enabling fill and finish capabilities and leveraging a wealth of expertise in componentry, devices, regulations and testing as an integrated solution to customers. The appetite for wearables is considerable and West will continue to grow and evolve, to predict and respond to the trends to help ensure our customers are providing up-to-date and innovative options for patients.
REFERENCES
- Pharmacircle, January 2019.
- IQVIA Data.
- US FDA CDER / CBER Data.
- De Cock E et al, “A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2- positive early breast cancer”. Cancer Med, 2016, Vol 5(3), pp 389-397.
- Jeroen J et al, “Fixed Dosing of Monoclonal Antibodies in Oncology”. The Oncologist, July 28, 2017.
Previous article
A QUICK GUIDE TO USABILITY FOR WEARABLE INJECTORSNext article
A VIAL-BASED SOLUTION FOR WEARABLE DRUG DELIVERY
