To Issue 171
Citation: “Understanding Patient Preferences for Sustainable On-Body Delivery Systems”, ONdrugDelivery, Issue 171 (Apr/May 2025), pp 22–24.
Stevanato Group examines how semi-reusable, modular designs like its Vertiva 10 mL on-body delivery system can reconcile patient demands for ease of use with growing expectations for sustainability, sharing new UK preference data and lifecycle analysis that reveal significant waste and emissions reductions.
Sustainability has become a critical priority for the healthcare sector due to the growing need to address the environmental impact of medical practices and devices. Globally, healthcare accounts for approximately 4.4% of greenhouse gas emissions, with single-use medical devices and pharmaceutical packaging being major contributors. For instance, the US generated over 5.9 million tons of medical waste in 2018, much of it being non-recyclable or hazardous.1
On-body delivery systems (OBDS), a rapidly expanding category of drug delivery devices suitable for a wide range of therapies, face similar challenges. As the healthcare industry strives to balance safety, efficacy and environmental responsibility, understanding patient preferences for sustainable solutions is essential.
“RESEARCH INDICATES THAT PATIENTS ARE BECOMING INCREASINGLY RECEPTIVE TO SUSTAINABLE HEALTHCARE SOLUTIONS, PROVIDED THAT THESE MAINTAIN USABILITY AND EFFICACY.”
PATIENT PREFERENCES FOR SUSTAINABILITY: INSIGHTS FROM LITERATURE
Research indicates that patients are becoming increasingly receptive to sustainable healthcare solutions, provided that these maintain usability and efficacy. For example, a 2020 survey conducted under the UK NHS sustainability agenda on asthma patients revealed that, while 65% were unaware of the carbon footprint of metered-dose inhalers, 60% would be willing to switch to greener alternatives, if ease of use and safety were not compromised.2
While such research on OBDS is still limited, early evidence is emerging. For instance, a 2024 human factors study on an OBDS in development that collected feedback from patients and healthcare professionals regarding various product attributes – including preferences for reusable versus single-use devices – found that 93% of participants preferred reusable OBDS. The primary motivations for this preference were the reduction of waste and plastic.3
Regional and cultural differences also influence preferences. According to a 2024 online survey that explored patient preferences for emerging injectable obesity therapies, patients in the UK, Germany and Switzerland demonstrated stronger preferences for reusable devices, reflecting greater environmental awareness. In contrast, US patients leaned toward single-use options, prioritising usability as their main concern.4
Interestingly, patients often associate sustainability with visible factors, such as reduced plastic usage, but may lack awareness of the environmental impact of different materials and components, such as electronics. For example, a 2023 online survey on patient perceptions of device sustainability found that single-use electromechanical OBDS were generally perceived as having a smaller environmental footprint than single-use mechanical autoinjectors.5 This perception likely stems from the reduced injection frequency that OBDS typically offer, rather than a comprehensive understanding of the environmental impacts of the materials and components used.
These findings highlight the importance of interconnected factors – such as user-centric design, regional preferences and material considerations – for driving patient adoption of sustainable solutions. Designing with these factors in mind offers a pathway to align sustainability with patient experience and treatment efficacy.
Stevanato Group’s Preference Study
As part of its commitment to aligning sustainability with usability in OBDS, Stevanato Group conducted a preference study in July 2024 with UK patients, focusing on its Vertiva® 10 mL, a semi-reusable OBDS designed to address both environmental concerns and patient needs. Vertiva’s modular design consists of two units (Figure 1):
- A reusable electromechanical controller, which powers the device and houses its electronic components
- A single-use injection unit, comprising only mechanical components and featuring a prefilled and preloaded drug cartridge.
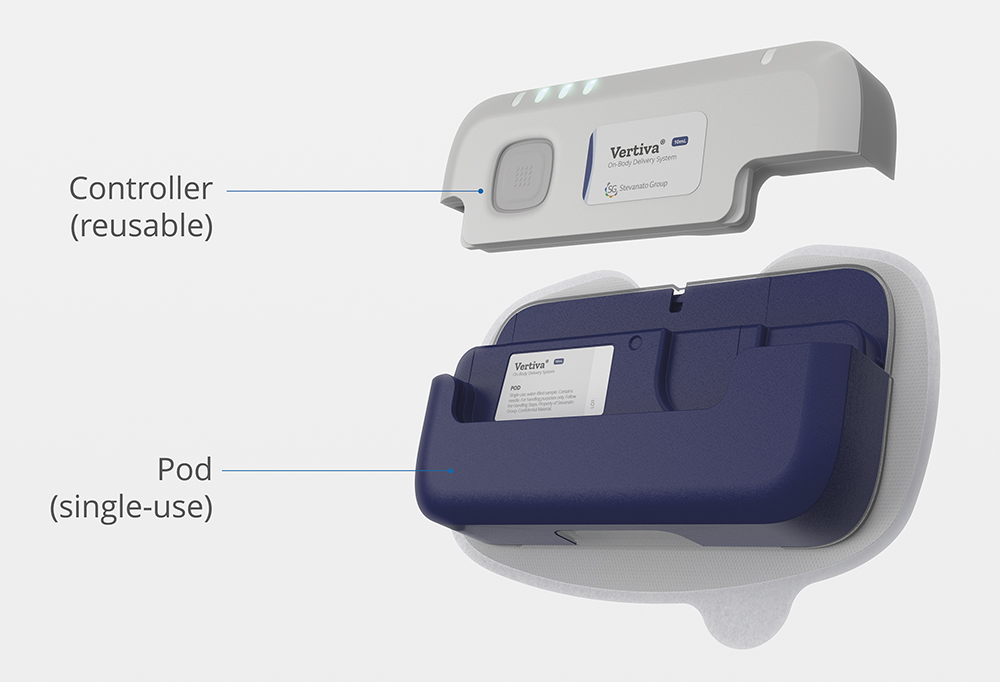
Figure 1: Stevanato Group’s Vertiva® 10 mL OBDS, featuring reusable electronics.
The study compared Vertiva with a fully disposable electromechanical OBDS. Unlike Vertiva, the comparison device involved discarding its electronics after each injection and required patients to manually load the drug cartridge before use. Both devices shared the same fill capacities and targeted similar therapeutic regimens, primarily differing in the presence or absence of a reusable module and the drug loading mechanism.

Figure 2: A patient completing the attribute assignation task during Stevanato Group’s Patient Preference Study.
Twelve participants, representing various demographics, medical conditions and familiarity with injection devices, reviewed mock devices, printed instructions for use and demonstration videos before evaluating the two devices. They first ranked a series of product attributes by importance, then matched each attribute to the device they felt best met the criterion (Figure 2), finally expressing their overall preference for one of the two devices.
Despite most patients emphasising the importance of environmental sustainability, it ranked fifth out of the seven evaluated product attributes on average, following ease of use steps, number of use steps, device size and ease of storage. This aligns with existing literature – while patients value sustainability, usability and convenience often take precedence.
Regarding attribute assignation, two-thirds of participants identified Vertiva as the more sustainable option, citing waste reduction thanks to its reusable unit. The remaining third perceived that both devices required disposal of a similar amount of plastic after each injection and therefore did not feel the reusable component offered a significant sustainability advantage. This also supports previous literature, which suggests that many patients equate sustainability with tangible design elements, such as reduced plastic usage, while overlooking the impact of less visible materials such as electronics (Box 1). This highlights the need for more effective communication from device manufacturers and pharmaceutical suppliers to address the gap in patient education and awareness of the broader issue of product sustainability.
BOX 1: QUOTES FROM PATIENTS
“I really like it. One of the things I feel somehow guilty about is the amount of plastic wastage, so I really like this idea of being able to reuse.”
“You keep the electronic part, that’s really good, because that saves a lot of plastic. Anyone with a chronic illness is aware of how much plastic waste there is.”
“I have loved that, with devices that have plastics and everything, they are making you retain some of them.”
Indeed, a 2023 lifecycle-analysis-based study assessing the environmental impact of a single-use electromechanical OBDS concept found that electronic components, such as processors and batteries, accounted for approximately 61% of the device’s total global warming potential, compared with just 16% for plastics.6 Based on these results, it is clear that reusable designs are key to mitigating the environmental impact of electronics. This was also found in a preliminary lifecycle analysis conducted by Stevanato Group on Vertiva, which compared emissions from its current semi-reusable design with those of a hypothetical single-use equivalent. The analysis found that emissions from device materials were nearly halved after just one reuse and reduced by approximately 70% after the fifth use (Figure 3). These findings underscore the potential of modular, reusable designs to significantly reduce the carbon footprint of a therapeutic regimen when electronics are involved.
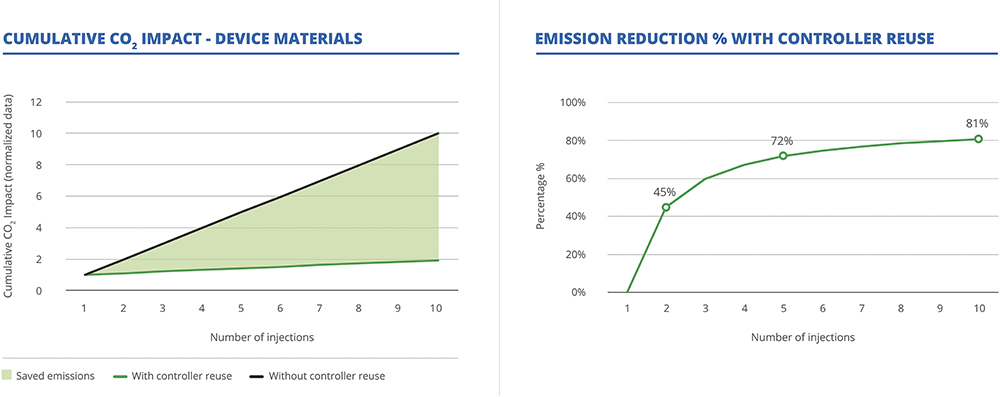
Figure 3: Preliminary material LCA on Vertiva® 3mL (analysis provided by an independent third party).
However, one key imperative remains – sustainability must not compromise patient safety or usability. Looking back at Stevanato Group’s preference study, Vertiva outperformed the comparison device in perceived usability, particularly in the ease of starting treatment. Over 80% of patients considered Vertiva easier to prepare, appreciating its preloaded and prefilled design, which eliminates the risks associated with manual drug cartridge loading. When asked about their overall device preference, eight out of 12 patients chose Vertiva, with most of these participants emphasising a perceived superiority in usability alongside its sustainability benefits. This result demonstrates that usability and sustainability can coexist when user-centric design principles guide product development.
CONCLUSION
Moving the healthcare sector towards sustainability requires a deep understanding of patient preferences to ensure adoption without compromising care. While studies consistently show that patients prioritise ease of use and reliability, sustainability is increasingly becoming a key differentiator. As the healthcare sector aligns more closely with the sustainability trends seen in other industries, its importance to patients is expected to grow even further.7
Insights from Stevanato Group’s research demonstrate how innovations like the Vertiva® 10 mL semi-reusable OBDS can address both patient needs and environmental concerns. By promoting modular designs, along with effective patient education and clear product communication, the industry can move towards a future where sustainability and patient care go hand in hand.
Don’t miss the chance to attend the PDA Miniverse, which takes place in Indianapolis, IN, US, on June 25 & 26, 2025, and which will include a tour of Stevanato Group’s new, state-of-the-art US hub. Register for the PDA Miniverse Conference, visit Stevanato Group at booth #218 and join us in Fishers (IN, US) on June 24: www.stevanatogroup.com/en/news-events/events/pda-miniverse-2025.
REFERENCES
- “Health Care’s Climate Footprint: How the Health Sector Contributes to the Global Climate Crisis and Opportunities for Action”. Health Care Without Harm, Sep 2019.
- D’Ancona G et al, “The sustainability agenda and inhaled therapy: what do patients want?”. Eur Respir J, 2021, Vol 58(Suppl 65), art PA3399.
- Bürdel S, Abedian R, “The Importance of User-Centric Design When Developing Devices for Home Use.” ONdrugDelivery, Issue 164 (Sep 2024), pp 36–40.
- Schneider A, “Decoding the Obesity Revolution: Mapping Device Needs and Patient Preferences for Emerging Injectable Obesity Therapies”. Presentation, PDA Universe of Pre-Filled Syringes and Injection Devices, Oct 2024.
- Lange J, “Patient Voices in Sustainable Drug Delivery: Insights on Self-Injection Devices”. Presentation, PDA Universe of Pre-Filled Syringes and Injection Devices, Oct 2023.
- Natarajan P, Bavar S, Dean C, “How Lifecycle Assessment Supports Insight-Driven Sustainable Design”. ONdrugDelivery, Issue 153 (Oct/Nov 2023), pp 16–19.
- “2024 Global Healthcare Sector Outlook: Navigating Transformation”. Report, Deloitte, Feb 2024.

