To Issue 178
Citation: Hasegawa H, Suzuki T, “Using Oxycapt™ Multilayer Plastic Vial for Gene and Cell Therapies Stored in Dry Ice and Liquid Nitrogen”. ONdrugDelivery, Issue 178 (Oct 2025), pp 134–139.
Hiroki Hasegawa and Tomohiro Suzuki consider the various challenges presented by cold-chain logistics to both traditional glass vials and many cyclo-olefin polymer alternatives, and how Mitsubishi Gas Chemical‘s OXYCAPT™ vials offer significant advantages for maintaining container closure integrity compared with other materials on the market.
OXYCAPT OVERVIEW
OXYCAPT™ is a multilayer plastic vial and syringe developed by Mitsubishi Gas Chemical (MGC), offering a number of advantageous qualities as a primary drug container, including:
- Excellent oxygen and ultraviolet (UV) light barrier
- Strong water vapour barrier
- Very low extractables
- High pH stability
- Low protein adsorption and aggregation
- High transparency
- High break resistance
- Easy disposability
- Lightweight material.

Figure 1: OXYCAPT multilayer plastic vial.
MGC continuously conducts studies to confirm these properties. The latest results of these are shared in the later part of the article. Before that, the first half of this article provides an overview of the OXYCAPT multilayer plastic vial (Figure 1). The material consists of three layers – the drug contact layer and the outer layer are made of cyclo-olefin polymer (COP) and the oxygen barrier layer is made of MGC’s novel polyester (Figure 2).
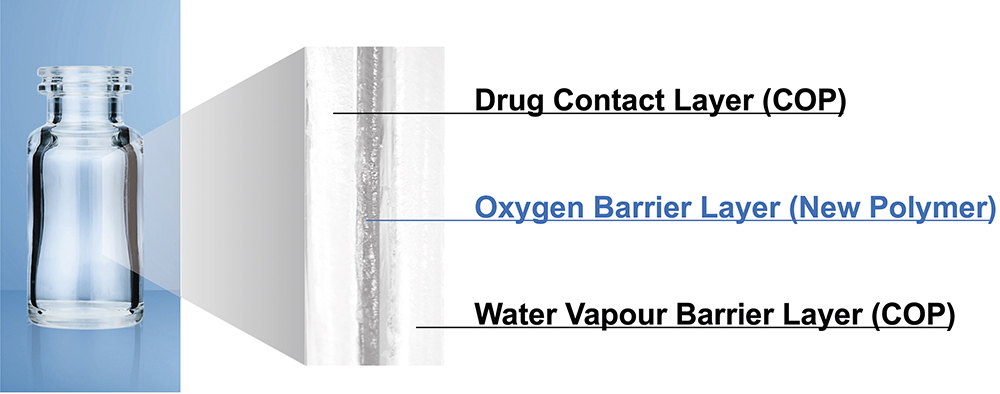
Figure 2: Multilayer structure of OXYCAPT.
MGC recently obtained a report on the environmental impact of glass and plastic containers for medical use from a Japanese research company. The report shows that plastic containers for medical use are much more environmentally friendly compared with glass containers. For example, the carbon footprint, nitrogen oxide emissions, sulphur oxide emissions and water consumption associated with plastic containers for medical use are several times smaller than those of their glass equivalents.
“OXYCAPT PROVIDES AN EXCELLENT OXYGEN BARRIER – FOR EXAMPLE, THE OXYGEN BARRIER OF AN OXYCAPT VIAL IS ABOUT 20 TIMES BETTER THAN THAT OF A COP MONOLAYER VIAL.”
OXYCAPT provides an excellent oxygen barrier – for example, the oxygen barrier of an OXYCAPT vial is about 20 times better than that of a COP monolayer vial. Furthermore, OXYCAPT provides an excellent UV barrier. While about 70% of 300 nm UV light transmits through glass and COP, only 1.7% transmits through OXYCAPT. MGC has confirmed that this feature contributes to the stability of biologics.
While OXYCAPT cannot reach the performance of glass with respect to acting as a water vapour barrier, its properties are similar to those of COP, which has been used for injectable drugs for a long time. This means that OXYCAPT easily meets the requirements of a water vapour barrier set out by the ICH guidelines.
Studies have shown an extremely low level of extractables from OXYCAPT. One study was conducted to confirm the levels of volatile, semi-volatile and non-volatile impurities from OXYCAPT. Water and four solutions (50% ethanol, sodium chloride, sodium hydroxide and phosphoric acid) were selected, and impurities were measured by gas chromatography mass spectrometry (GC-MS) and liquid chromatography-UV spectroscopy-mass spectrometry (LC-UV-MS) after 70 days at 40°C. Compared with the control, impurities were not detected in the OXYCAPT containers. A second study confirmed that inorganic extractables levels from OXYCAPT were similar to those from COP, which is well known for being an extremely pure polymer with a better extractables profile than Type 1 glass. Lower levels of inorganic extractables are known to contribute to better pH stability in drug products.
The OXYCAPT vial is produced by co-injection blow-moulding technology. MGC has also developed inspection methods for testing the oxygen barrier layer. All the containers are fully inspected by state-of-the-art inspection machinery.
MGC can offer bulk vials and ready-to-use (RTU) vials, with its RTU products provided in standard nest-and-tub or tray formats. The nest and tub are mainly sterilised using gamma rays. There are 2, 6, 10 and 20 mL variants for vials. MGC is willing to provide samples for initial testing free of charge.
Each polymer meets the requirements of US Pharmacopeia (USP) regulations USP <661>, USP <87> and USP <88>, as well as those of the European Pharmacopoeia, and has been filed in the US FDA’s drug master file (DMF). The vials are also compliant with each pharmacopoeia and have been filed in the DMF.
The primary target market for OXYCAPT is the therapeutic application of biologics. As mentioned in ICH Q5C (Stability of Biotechnological/Biological Products), oxidation is one of the causes of protein instability. As such, the oxygen and UV barrier properties of OXYCAPT contribute to the stability of biologics stored within. Furthermore, some drug developers have recently started evaluating OXYCAPT vials for their gene and cell therapies; the RTU vial is sterilised by gamma radiation, making it ideal for protein-based drugs.
“OXYCAPT VIALS DEMONSTRATED EXCELLENT PERFORMANCE IN A HEAT CYCLING TEST DESIGNED TO EVALUATE RESISTANCE TO PHYSICAL STRESSES SUCH AS MATERIAL CONTRACTION AND EXPANSION, AS WELL AS THE VOLUMETRIC EXPANSION OF AQUEOUS DRUG FORMULATIONS.”
OXYCAPT AT ULTRA-LOW TEMPERATURES
Plastic vials generally exhibit greater toughness at ultra-low temperatures compared with glass, markedly mitigating the risk of breakage during transportation and under thermal stress. For example, OXYCAPT vials demonstrated excellent performance in a heat cycling test designed to evaluate resistance to physical stresses such as material contraction and expansion, as well as the volumetric expansion of aqueous drug formulations. In this study, 8 mL of a 10 w/v% mannitol aqueous solution was filled into 20 10R OXYCAPT vials and 20 conventional glass vials, which were then sealed with rubber stoppers. The vials were alternately placed in constant-temperature chambers set at -80°C and 40°C, with each cycle consisting of storage at both temperatures for three to four consecutive days. This alternating process was repeated for a total of five cycles. By the end of the fifth cycle, all 20 glass vials were broken, whereas none of the 20 OXYCAPT™ vials exhibited any visible defects, such as cracks or layer separation (Table 1).
| Vial | Total Number of Breakages | Total Number of Layer-Separations | ||||||||
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | Cycle 5 | |
| OXYCAPT™-P V10 RT | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 | 0/20 |
| Competitor’s Glass Vial(10R) | 5/20 | 10/20 | 14/20 | 20/20 | – | N/A | ||||
Table 1: Heat cycling stress test performed with mannitol aqueous solution.
Container Closure Integrity
State-of-the-art drug products, including messenger RNA (mRNA)-based drugs and gene and cell therapies, are typically transported and stored at ultra-low temperature conditions (-78°C) using dry ice or at cryogenic temperature (-180°C) in the vapour phase of liquid nitrogen. Under these circumstances, there is a risk of losing container closure integrity (CCI), particularly for glass vials. This risk arises from the remarkable difference in the coefficients of thermal expansion between the vial’s glass flange and the elastomeric closure.
To simulate the conditions under which gene and cell therapy products are transported and stored, MGC conducted a CCI study using OXYCAPT vials. In this study, OXYCAPT vials and conventional glass vials were prepared, sealed under ambient air with non-laminated bromobutyl rubber stoppers and aluminium caps, after which their initial oxygen concentration was measured using an FMS-760, a headspace oxygen analyser (LIGHTHOUSE Instruments, Charlottesville, VA, US).
The vials were then stored in the vapour phase of liquid nitrogen within a dry shipper for up to three months. During storage, the oxygen concentration inside the vials was periodically measured to verify whether or not it remained at approximately 20.9%. The results showed that all of the conventional glass vials lost CCI within seven days, whereas the OXYCAPT vials maintained CCI even after three months (Figure 3). These findings demonstrate that OXYCAPT vials effectively prevent the ingress of air, moisture and contaminants, even under cryogenic conditions.
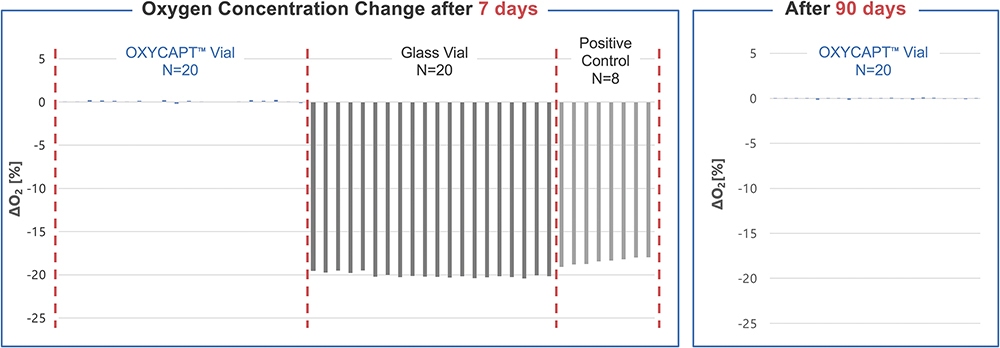
Figure 3: CCI test results in vapour phase of liquid nitrogen.
Risk of Carbon Dioxide Ingress
While plastic vials are capable of markedly mitigating the risk of loss of CCI, conventional plastic vials present an alternative inherent risk under conditions of coexisting dry ice – the permeation of carbon dioxide (CO2). To elucidate this latent risk, a simulated study was conducted using dry ice, as illustrated in Figure 4.
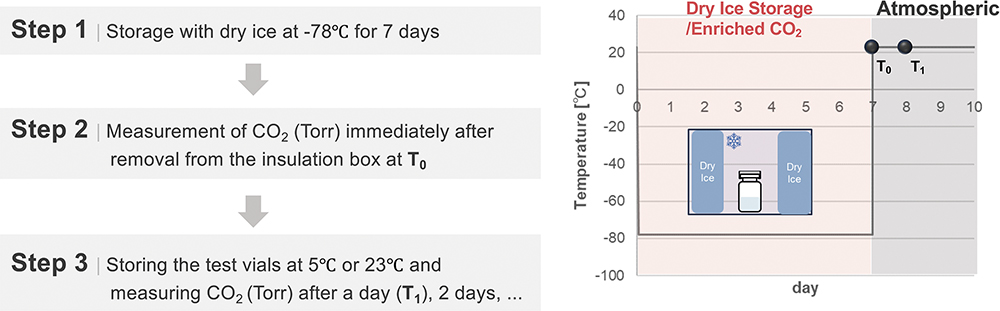
Figure 4: Environmental profile during CCI test with dry ice.
Five 10R OXYCAPT vials and five conventional COP vials were used as test samples. The vials were sealed with commercially available bromobutyl rubber closures and aluminium caps under a 100% nitrogen atmosphere. The vials were placed in an insulated container filled with dry ice and stored for seven days. Immediately upon removal (T0), the CO2 partial pressure in the vial headspaces was measured using FMS-CO2 (LIGHTHOUSE Instruments). The vials were then stored under ambient conditions at either 5°C or 23°C, and the CO2 partial pressure was measured again after one day, with subsequent periodic measurements performed over time.
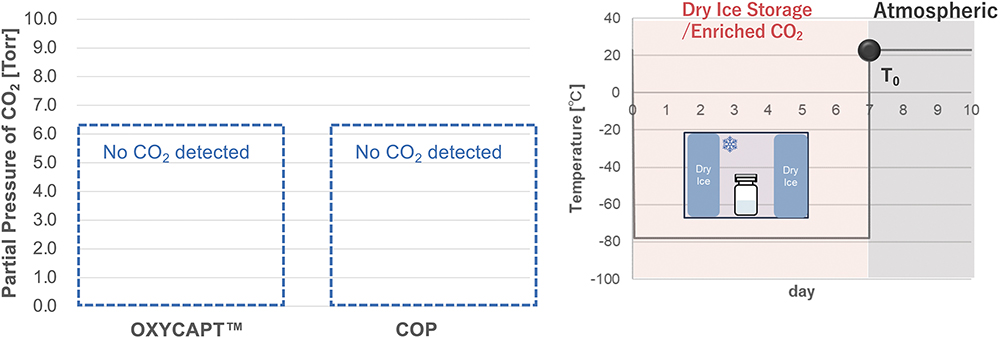
Figure 5: CO2 measurements in vial headspace immediately after removal.
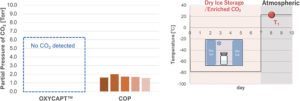
Figure 6: CO2 measurements in vial headspace one day after removal.
As shown in Figure 5, no CO2 was detected in the headspaces of either the COP or OXYCAPT vials at T0, demonstrating excellent CCI at -78°C. However, when subsequently stored at 23°C for one day, the COP vials exhibited a CO2 partial pressure of approximately 1.5 Torr (Figure 6). This phenomenon was also observed when the COP vials were stored at 5°C. In contrast, the OXYCAPT vials maintained a CO2 concentration of 0% over a period of 70 days, even under continuous storage at 23°C (Figure 7).
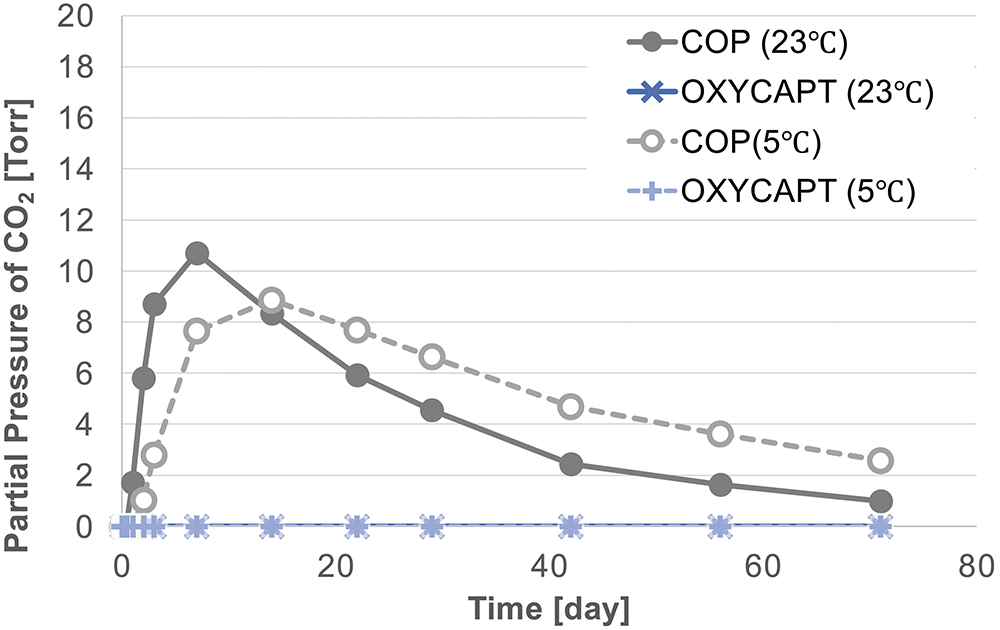
Figure 7: Changes in CO2 partial pressure in vial headspaces after removal from insulated chamber.
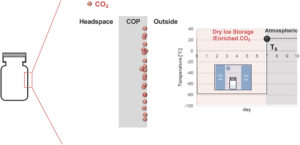
Figure 8: Schematic illustration of CO2 dissolution in COP vials immediately after removal from dry ice.
This time-dependent permeation phenomenon of CO2 can be attributed to the inherent physical properties of CO2, which exhibits relatively high solubility coefficients at ultra-low temperatures and is readily soluble even in plastics. During cold storage, the CO2 generated through the sublimation of dry ice dissolves into the near-surface region of the polymer layer. Under ultra-low temperature conditions, gas molecules scarcely diffuse through the polymer layer, meaning that, even when the headspace CO2 partial pressure was measured immediately after removal from cold storage, no detection was observed (Figure 8). In contrast, upon subsequent storage under comparatively mild conditions, such as room temperature or 2–8°C, the dissolved CO2 rapidly diffuses through the polymer and reaches the vial headspace (Figure 9).
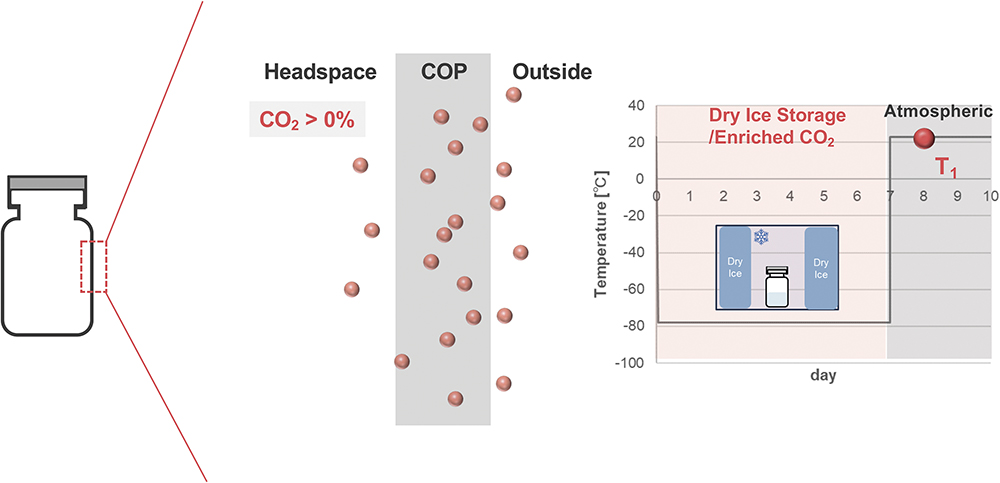
Figure 9: Schematic illustration of CO2 diffusion through COP vials one day after removal.
An additional verification study was conducted using vials filled with distilled water, in which the pH was measured following storage with dry ice. Thirty samples were prepared for each vial type – 10R OXYCAPT, COP and glass. The vials were filled with distilled water under a nitrogen atmosphere and the initial pH value was measured at T0 before sealing the vials with commercially available bromobutyl rubber stoppers (non-laminate). The samples were then divided into two groups – 15 vials were stored for seven days in the presence of dry ice and 15 vials were stored in a -80°C deep freezer. After storage, five vials from each group were thawed at 23°C for eight hours, after which the pH was measured again (T7). Additional destructive testing was performed on independent sets of five vials each at one day (T7+1) and four days (T7+4) after thawing, with each vial discarded following measurement. The results showed that, in the COP vials stored with dry ice, the pH decreased beginning at T7+1. In contrast, the OXYCAPT vials maintained their initial pH even at T7+4 (Figure 10).
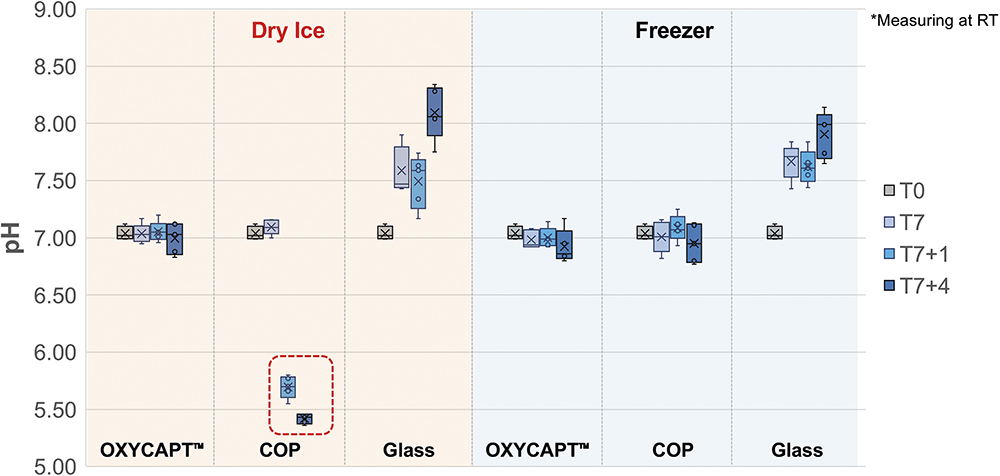
Figure 10: pH variation of distilled water after dry ice storage.
In practice, upon delivery, pharmaceuticals transported with dry ice may be thawed or temporarily refrigerated at 2–8°C prior to administration. Under such conditions, exposure to ambient or refrigerated temperatures can result in the permeation of dissolved CO2. Once present, CO2 dissolves in aqueous drug formulations, generating protons and therefore posing a latent risk of detrimental pH shift in drug formulation.
To mitigate this “time-delayed” CO2 permeation phenomenon, secondary packaging is sometimes used. However, secondary packaging introduces additional concerns, including the risk of physical damage, increased material and logistical costs and the necessity for packaging validation. In view of these challenges, OXYCAPT, which provides inherent gas-barrier properties at the level of the primary container, represents an optimal solution by ensuring CCI while simultaneously offering advantages in cost efficiency and environmental sustainability.

