Citation: Carty N, Prakash D, “Expert View: Wearable Injectors: Choosing and Testing Adhesives for Optimal Performance”. ONdrugDelivery Magazine, Issue 100 (Sep 2019), pp 20-23.
Neal Carty and Deepak Prakash highlight the importance of choosing and testing adhesives for optimal wearable drug delivery device performance.
Wearable drug delivery devices are a compelling area of medical device and pharmaceutical industry development. Innovations already on the market are enhancing patients’ quality of life, and many developments in the pipeline have the potential to deliver increasing levels of comfort, convenience and lowered healthcare costs.
These devices have diverse applications, from closed-loop continuous glucose monitoring (CGM)/insulin delivery systems to wearable large-volume injectors (LVIs) for delivery of a growing number of biologics and other drugs.
LVIs, in particular, are gaining attention for enabling patients to self-administer novel medications for chronic diseases in the home. More than 50% of the global population suffers from a chronic disease, according to a Roots Analysis report citing data from the University of Michigan’s Center for Managing Chronic Disease. A prevalent way to administer many newer biologic drugs for chronic diseases is through intravenous (IV) injection or infusion in the hospital or other clinical setting – but this has drawbacks.
“Wearable devices have wide-ranging wear time requirements and performance specifications, so there is no “one size fits all” when it comes to adhesive selection.”
“The majority of the available treatment options require parenteral administration, frequent dosing, involve repeated hospital visits and are associated with multiple other concerns, such as dosing and medication errors, risk of microbial contamination and needlestick injuries. These challenges represent a substantial threat to medication adherence and, thereby, are likely to significantly impact therapeutic outcomes,” said Roots Analysis in the overview to its December 2018 report.1
“Over the past few years, a number of companies have developed advanced therapeutic delivery solutions to alleviate the pressing concerns associated with the administration of both conventional and novel drug / therapy molecules,” according to Roots Analysis. “Amongst modern drug delivery practices, the concept of self-injection has facilitated advanced medications to be administered beyond the clinical setting. This has also served to reduce healthcare costs, improve therapy adherence and optimise the utilisation of healthcare resources per treatment.”
Michael Hooven, President and Chief Executive Officer of Enable Injections, noted that there are more than 2,700 biologics in development, including new treatments for cancer, autoimmune disease, neurologic disease and other diseases. And that researchers have found that their economic value may depend on their cost of administration.2
Prescient & Strategic Intelligence (P&S) has estimated the wearable injector market will grow from US$2.16 billion (£1.78 billion) in 2015 to $13 billion in 2024. P&S has predicted cancer treatments will become the largest application area. Other application areas expected to experience growth include treatments for autoimmune, infectious and cardiovascular diseases, and blood disorders. “Growing need for advanced drug delivery is one of the major factors driving the growth of the global wearable injectors market. Increasing demand for home care and advanced drug delivery requiring minimal expertise and lesser hospitalisation of patients is expected to drive the demand for wearable injectors, globally,” P&S said in an October 2017 blog post.3 “Also, many biopharmaceutical companies have been looking for a better mode of drug delivery for their portfolio of biologic drugs, for which wearable injectors are an appropriate solution.”
In many of the same ways that LVIs are making life easier for patients with various chronic diseases, integrated CGM solutions have been game changers in the daily quality of life for patients with diabetes. Patients using closed-loop CGM systems no longer have to check their blood glucose levels multiple times per day. Instead, the CGM solution automatically monitors glucose levels in their interstitial tissue just below their skin surface and adjusts their insulin flow accordingly.
Abbott, maker of the FreeStyle Libre CGM system, discussed during its second quarter 2019 earnings call4 that the Libre technology has “enormous potential” beyond monitoring glucose. It remains to be seen how this type of continuous monitoring solution, integrated with drug delivery devices, will evolve into other disease categories but the potential is exciting.
Many of the latest CGM and LVI solutions offer connectivity to smartphone apps so that patients and, if desired, their support networks can stay apprised of their medication status, make sure doses have been delivered successfully and receive reminders.
“Connectivity between sensors and devices is enabling healthcare organisations to streamline their clinical operations and workflow management, and improve patient care, even from remote locations,” said the Deloitte Centre for Health Solutions in a 2018 report.5 “Provided medtech companies can convince clinicians and patients of the value and benefits of connected medical devices, the pace and scale of healthcare transformation will be exponential.”
ADHESIVE SELECTION CONSIDERATIONS
The “wearable” in wearable drug delivery devices is often enabled by adhering the device directly to the body with a medical-grade pressure-sensitive adhesive (PSA) material (Figure 1). Wearable devices have wide-ranging wear time requirements and performance specifications, so there is no “one size fits all” when it comes to adhesive selection. For example, the body-worn portion of an integrated CGM system may consist of a half-inch diameter patch that needs to stay on for 15-30 days. On the other hand, there are much larger LVI devices designed to deliver anywhere from 2-50 mL of medication, and they may need to remain on the body for 15-30 minutes or a few days.
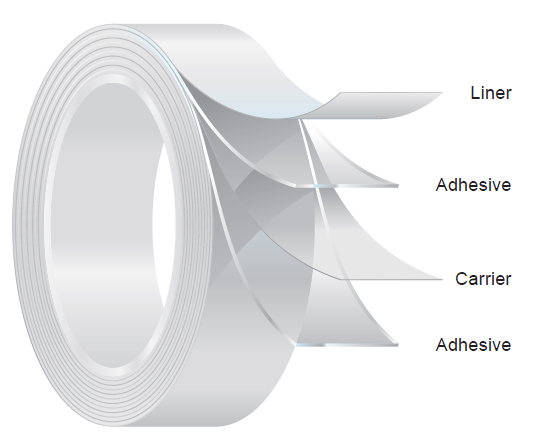
Figure 1: The construction of a double-coated PSA medical tape. This type of tape is commonly used in wearable medical devices.
Regardless of the wear time duration, the devices generally need to withstand a patient’s normal daily life activities and movements. For extended wear devices, this often includes showering and exercise. The PSA material should also allow for easy fixation of the device to the body and atraumatic removal. With these goals in mind, the following are some adhesive factors to consider.
Moisture Management
Moisture management and patient comfort go hand in hand. A wearable device’s skin-contact layer adhesive and any construction-layer adhesives should offer an effective means of removing perspiration and moisture that inevitably will present on the patient’s skin. The two primary methods of moisture management are fluid absorption (being absorbed and contained in the material) and vapour transmission (being evaporated through pores in the material).
Related questions to consider:
- What is the average perspiration rate for the body location where the device will be worn during different levels of physical activity?
- Will the patient be exposing the device to moisture through bathing, exercising or other daily life activities?
Static Shear
The static shear, or cohesion, of an adhesive is its ability to hold in position through shearing forces, such as stretching, bending and twisting of the body.
Related questions to consider:
- How long will the device be worn and what activities will the patient likely perform during this time?
- Is the body location for the device one that experiences frequent twisting, stretching or bending?
Peel
The peel, or peel adherence, of an adhesive is its ability to resist removal by peeling. Some peel adhesion tests measure how much force is required to peel the adhesive from a certain substrate.
Related questions to consider:
- Will the typical device end user have delicate, fragile skin?
- How large and heavy is the device, and how long must it be worn? How strong does the peel adherence need to be?
Tack
An adhesive’s tack determines how quickly it sticks, or adheres. A high tack level may mean that the adhesive adheres almost instantly. Others may need to be held under light pressure for a little while to be secured.
Related questions to consider:
- Is it likely the drug delivery device end user will need to be able to reposition the device if he or she misses the desired application position on the first attempt?
- Will the device be applied to the body in a location that allows the patient to easily hold it in place for several seconds with gentle pressure for proper securement?
Biocompatibility
Adhesive materials that are highly biocompatible are less likely to cause irritation or allergic reaction when adhered to the patient’s skin. At a minimum, these adhesives have passed the ISO 10993 standard tests for cytotoxicity, irritation and sensitivity.
Related questions to consider:
- In addition to ISO 10993, have the adhesive materials been engineered to meet localised biocompatibility requirements for different geographic markets?
- Can the supplier address specific allergen concerns relative to the device application?
EARLY CONCEPT WEAR TESTING
“It’s crucial for every material and component to play
its supporting role perfectly, otherwise patients may not
realise the full benefit of their therapy.”
Each of the adhesive characteristics described above can and should be tested in a laboratory using industry-recognised, standardised testing methods, and these tests and their results are very important. However, laboratory testing can only tell a device developer so much about how a human being will respond to a body-worn product. Before locking in an adhesive material choice or freezing the device design, wear testing of early concepts can help with decisions about what materials and features to carry forward into product development.
Wear tests conducted early in the product development cycle can provide an opportunity to discover important end user reactions and perhaps dispel some erroneous assumptions. Concept drawings frequently take a naïve view of how a wearable device may fit on an idealised human form. But through user testing on real human subjects we can discover how well a device actually works with a diversity of different body types. For example, women may find a wearable device to be far more uncomfortable or impractical than male subjects for a reason as simple as a difference in how undergarments or a purse strap rub the device in an irritating way. Human bodies come in an incredibly diverse set of shapes and sizes, and that diversity’s impact on product performance should not be underestimated. Wear testing can reveal how a wearable product concept really looks and feels on a variety of people of different ages and with different body masses, skin types and behaviours.
Often, these early concept wear tests can be performed and results analysed within a relatively quick time-frame, so device developers do not need to worry about losing months of cycle time for such testing. By investing time early on for wear testing, the device maker might avoid time-consuming setbacks later in product development and regulatory review.
CONCLUSIONS
Wearable drug delivery devices represent a fast-growing segment of the healthcare industry. Innovations in this space provide a key link in a future focused on more connected, accessible and economical healthcare solutions. While the pharmaceutical drug inside these devices is unquestionably the most valuable aspect of the system, it’s crucial for every material and component to play its supporting role perfectly, otherwise patients may not realise the full benefit of their therapy.
Often the primary point of contact between the device and the patient’s body, advanced medical PSA, is instrumental in device securement and patient comfort. Device construction, moisture management and wear location can affect manufacturability, wear time and the patient experience.
REFERENCES
- Roots Analysis, “Large Volume Wearable Injectors Market (4th Edition), 2018-2030”. 2018.
- Hooven S, “Large-Volume Wearable Injectors: A New Delivery Approach Could Change the Game”. BioProcess International, 2018, Vol 16(5).
- Prescient & Strategic (P&S) Intelligence Private Ltd, “Bolus Injectors Contribute Largest Revenue to Global Wearable Injector Market”. P&S blog, 2017.
- Muoio D, “Abbott ramping up FreeStyle Libre production following bountiful Q2”. MobiHealth News, 2019.
- Ronte H, Taylor K, Haughey J, “Medtech and the Internet of Medical Things: How connected medical devices are transforming health care”. Deloitte website, 2018.

