Citation: Costantino C, Fennelly L, “Expert View: A Quick Guide to Usability for Wearable Injectors. ONdrugDelivery Magazine, Issue 100 (Sep 2019), pp 49-53.
Cory Costantino and Lauren Fennelly list some typical steps a user might take while using wearable injectors, providing tips and points to note at each step, including design tips that might pre-empt potential interaction problems, and points to consider when conducting usability testing.
INTRODUCTION
Although there are a wide variety of wearable injectors, there are also many common characteristics that impact the user’s experience. Here we present a Quick Guide list of some of the trends we’ve observed supporting the design and usability testing of these products. For each step, we provide our insights on potential usability issues to consider when developing and testing a wearable injector’s design (see Figure 1).
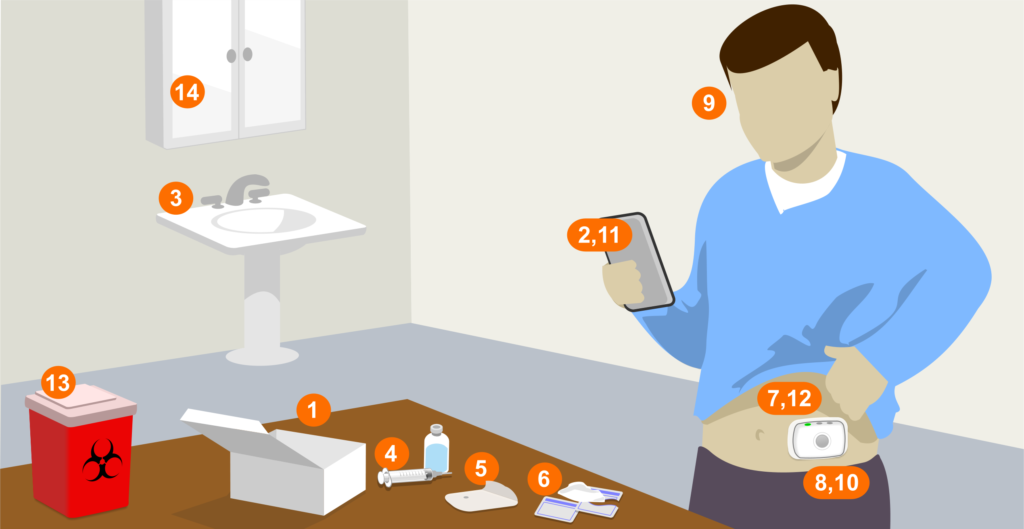
Figure 1: Patients can face many opportunities for interactive problems when working with wearable injectors. Numbers in the figure above correspond with the steps outlined in this article.
1. GATHER SUPPLIES
“Newer users will likely feel apprehensive as they position the device onto their skin, focusing on the startling needle stick they’ll feel in just a few moments rather than the task at hand. However, device design can help account for
users’ misplaced focus.”
Gathering essentials such as alcohol swabs, hand sanitiser, the device, and medication onto a clean surface might seem like an obvious and simple first step. But, there can be some critical choices to make.
- Drug differentiation. Users might have to select among multiple drugs for use in the device. Legible, conspicuous labelling on the medication cartridges, vials, syringes, and other accessories will help users avoid selecting the wrong medication or concentration.
- Dose/Device differentiation. To support easy differentiation when devices are available in different volume capacities (and prevent incorrect dosing), each device’s carton and labelling on the device itself should display its capacity in large text (e.g. 14-18 pt) and different coloured labels. Better yet, the devices themselves could be different colours or sizes to make visual distinction even more emphatic.
Testing tip: Provide adequate workspace needed to accommodate supplies. Supplies on a neatly set test room table can double or triple in footprint when participants remove inner packaging, drug vials, blister packs, and unfold the IFU (see Table 1 for a summary of the testing tips).
| Ensure adequate workspace for participants |
| Include a representative patient sample, including a variety of ages (if applicable to condition) |
| Video record key steps (such as filling the device) during the session |
| Exercise human subjects protection practices (especially if device will be applied to skin) |
| Intervene before a needlestick injury occurs |
| Use a “cooking show” format to simulate time passing |
| Include scenarios with and without controller component |
Table 1: Key tips for conducting usability tests of wearable injectors.
2. PAIR DEVICE WITH CONTROLLER OR APP
Some wearable injectors feature a controller or app that enables the user to program an injection, view dose history, or adjust other device settings.
- Simple. Pairing and setting up connected devices can seem cumbersome and confusing, particularly to less tech-savvy users. Seek ways to simplify the process, for example separate the task of pairing the controller and device from other setup steps like creating accounts and connecting to networks.
- In sync. Ideally, the controller will be able to detect where the user is in the workflow, and be forgiving when users perform steps out of order (and provide support to get the user back on track).
- Age appropriate. Consider how young adults might be comfortable using a controller or app along with the device, whereas elderly patients might be more resistant to a screen-based paradigm.
Testing tip: Ensure the people in your usability tests – the participant sample – reflect the age range of people who have the condition(s) the product is designed to treat.
3. WASH HANDS
For all injection devices, washing hands is a critical step to preventing infection. Although cleanliness can be subjective, manufacturers should include helpful graphics in an instructional step to help patients remember this step and establish good habits.
4. FILL THE DEVICE
For myriad reasons, it’s not always possible to provide a prefilled device. In this case, simplifying the device filling process is the next best thing.
- Filling tools. Using a standard syringe to fill a device can be a difficult task. Users face challenges such as handling delicate/sharp needles, viewing small graduation lines, reading the meniscus, pushing against high-viscosity drugs or back pressure, and targeting small, inconspicuous fill ports. In general, filling accessories should aim to minimise these challenges and demands on users’ dexterity. A Luer lock filling port, for example, rather than a small port that must be accessed by a needle, could be a simple improvement.
- Fill location. We often see users struggle to find the fill port. This can happen because the fill port closely resembles other small, neutral colour device features, including indicator lights, vents, or the plastic housing. Conspicuous graphic icons (e.g. a syringe icon printed on the adhesive liner) can help users differentiate the fill port from other device features.
- When to fill. Some wearable injectors require users to fill the device before interacting with other system components. In such cases, on-device labelling – such as stickers preventing the user from moving a specific component – can be an effective method to prevent filling at the wrong time.
Testing tip: Focus a tripod-mounted HD camera closely on the patient’s hands during filling tasks. Analyse this footage retroactively to pinpoint filling interactions that are particularly challenging for users.
5. REMOVE ADHESIVE LINER
Adhesive liners with inadequate gripping area can result in tearing or wrinkling the adhesive, resulting in compromised contact with the patient’s skin. Ensure the adhesive liner has adequately large pull tabs and separates easily from the adhesive pad – especially when designing for patients with reduced visual acuity or dexterity impairments.
6. PREPARE INJECTION SITE
“Ideally, devices have a small footprint and low profile
with rounded edges. This helps ensure comfort in a
variety of body locations, and reduces the likelihood
of the device bumping into objects in the environment
or getting caught in the user’s clothing.”
Similar to hand-washing, patients have a broad range of cleanliness standards. Instructional materials should include helpful graphics to help patients establish good habits.
Testing tip: Some individuals might have adhesive allergies or be uncomfortable exposing skin during the test session. If test participants must place the device on the skin (versus on simulated skin), inform potential participants during the screening and consent process.
7. PLACE DEVICE ON BODY
Newer users will likely feel apprehensive as they position the device onto their skin, focusing on the startling needle stick they’ll feel in just a few moments rather than the task at hand. However, device design can help account for users’ misplaced focus.
- Location. Instructional materials should use simple anatomical graphics with enough context to help users identify suitable body locations for a wearable device.
- Orientation. Users might place a device in an incorrect orientation. Ideally, the device design enables the user to access critical controls and view critical indicators irrespective of orientation or location on the body. Regardless, always provide clear instructions and very conspicuous orientation markings to guide proper placement.
- Comfortable design. Users should feel comfortable wearing the device, and confident that the device will remain secure. Ideally, devices have a small footprint and low profile with rounded edges. This helps ensure comfort in a variety of body locations, and reduces the likelihood of the device bumping into objects in the environment or getting caught in the user’s clothing.
8. ACTIVATE INJECTOR AND START INJECTION
“While it might seem opportune to present information (screens, LEDs, dose windows) on the device’s largest flat surface, that surface is often perpendicular to the user’s line of sight, given that the larger surface would have the adhesive patch. Seek opportunities to place key information on sides that are in direct line of sight, which might be the shorter, protruding side of the device.”
Although the need to insert the needle and start injection is arguably the most obvious and anticipated step for the user, there are still opportunities where use error can confound their efforts.
- Unlock. An unlock step can provide a simple means of blocking mechanical, electrical, or chemical functions from occurring inadvertently until the time of use. If included, ensure such features are salient and simple, such as a button with “unlock” icon, a one-way twisting motion, or a coloured pull tab or cap.
- Power on. Users might expect to have to power-on a wearable injector that includes a screen and buttons before using it. However, if the device is relatively simple (e.g. a single-use device) users might have difficulty determining if the device first needs to be powered-on, then started, or simply started in one action. In either case, ensure that the device clearly distinguishes between “on” and “start” if both steps are needed.
- Inadvertent activation. Preparation steps can require varying degrees of handling, which could lead a user to press a start button inadvertently before applying the device to their body. Such a use error can waste valuable medication, breach sterility, or cause needle-stick injuries. Explore opportunities to protect the start button with a protruding rim or unlock feature.
- Needle awareness. Ensure that the device provides clear graphics and features indicating the needle’s location so users keep their hands away from the needle’s path, and position the device properly. Even if the injector includes a sensor to detect proper skin contact before allowing the needle to deploy, consider whether a user could inadvertently activate the sensor when handling the device.
Testing tip: If there is potential for needle-stick injury, inform participants before starting the test session. The moderator can intervene if they think a participant is about to get a needle stick.
9. MONITOR INJECTION
If users need to check on their injection status or if the device needs to get the user’s attention (e.g. due to an occlusion), the device should clearly indicate its status in a passive or assertive manner, respectively.
- User’s perspective. While it might seem opportune to present information (screens, LEDs, dose windows) on the device’s largest flat surface, that surface is often perpendicular to the user’s line of sight, given that the larger surface would have the adhesive patch. Seek opportunities to place key information on sides that are in direct line of sight, which might be the shorter, protruding side of the device.
- Infusion duration. Some wearable injector products might infuse drug over several minutes and only be used once a week or once every few weeks, while others might infuse drug for longer periods. Assume that devices delivering longer infusions might be subjected to more varied use environments and user activities, and those with longer durations between infusions might stretch the user’s ability to remember the proper injection workflow.
Testing tip: Longer infusion times can pose a challenge for typical usability test durations. Consider using a “cooking show” format during testing: participants prepare and start an injection, then, after a short period, the test moderator artificially triggers the end of infusion, and the participant resumes as they would at the end of infusion.
- Labels. During an injection, static labels can offer an effective gauge of infusion progress. For example, legible syringe barrel graduations against a high-contrast plunger can create a simple, effective gauge. Similar inspiration might come from an automobile fuel gauge, where a pointer moves along the scale from full to empty.
- Signals. While audible and vibratory signals might gain users’ attention, users will likely seek visual confirmation for a device-related event. Therefore, ensure the haptic and audio feedback is intense enough to be detected and prompt inspection, but also provide intuitive visual signals to confirm the current status. Additionally, consider limiting the number of distinct audio and haptic feedback signals, recognising that users might have difficulty distinguishing between them.
- Controllers and Quick Reference Guides. Separate controllers, apps and quick guides can offer more details about the current injection status. For example, a controller might provide real-time status information and provide instructions on assessing and resolving an alert state.
Testing tip: If the wearable injector system includes a separate controller, include use scenarios that replicate system use with and without the controller to evaluate whether users can effectively use or monitor the device without the controller.
10. ADJUST THERAPY DURING INFUSION
Some wearable injectors enable setting adjustments during infusion. To do so, the device might offer physical buttons or functions on a controller.
- Quick access. Some wearable injectors include quick-access functions to adjust the therapy. For example, for a quick insulin bolus, users might have to remember how much insulin each press delivers, detect successful button actuation, and keep track of how many times they pressed the button. Such physical and cognitive demands can be overwhelming, leading to use errors.
- Physical action. Designing a button to be used on a soft, moving surface (i.e. the body) is inherently difficult. A button on the front surface might require the user to push the button into themselves, while a button on a side surface might require squeezing around the overall device for leverage. In either case, protect buttons with an unlock, recess, or raised lip to prevent accidental actuation. Importantly, consider users’ hand sizes and grip strength, and provide an appropriate travel distance (~3 mm) and feedback (such as an audible and physical click) so that users can detect when they’ve successfully actuated the button.
- Controllers. Apps could mitigate some concerns related to quick access and physical buttons. When providing controls via an app, clearly present the device’s status and most likely-used controls on screen (e.g. bolus function, pause therapy, increase/decrease infusion rate), while relegating other functions and information to a menu or secondary screen.
11. CONFIRM COMPLETED INJECTION
Users will want to know when the injection is complete. A device simply turning off upon completion is not only anticlimactic, but also flawed user interface design – users might not be able to determine whether the injection completed successfully or failed in the midst of infusion.
A controller screen should be able to provide a clear message, but the device should indicate its status as well. The device could provide haptic and audible feedback to get the user’s attention, but it should also provide a visual signal. For example, a green LED to indicate “complete” (assuming that the same green light does not also indicate a “start” or “in progress” condition). Moreover, a physical mechanism – such as a plunger or gauge that changes colour, position, and labelling when complete – can create a unique physical “done” state.
12. REMOVE WEARABLE INJECTOR
Most wearable injectors are removed by peeling away the device. Make sure there is enough adhesive patch protruding from the device for users to grasp the patch’s edge and get leverage to peel it away. In addition, when the injection is complete, the needle should retract into the device to reduce discomfort during removal and prevent a needle-stick injury.
13. DISPOSAL
Users might be unsure how to dispose of their wearable injector properly, especially when devices have a retracted (i.e. not visible) needle. Provide clear instructions for proper disposal.
- Single-use components. Users can be tempted to reuse single-use components due to the cost of some medications. Ideally, design all single-use components to prevent reuse. Provide training and explanation in instructional material regarding the risks of reusing components.
- Durable components. Inadvertently discarding a durable component can be costly and delay therapy. Components with a screen or buttons might be more easily recognised as reusable, but, in general, design durable components to be visually distinct, appealing, and dominant in appearance to help convey they should be retained after completing the injection.
14. STORE WEARABLE INJECTOR AND SUPPLIES
Storage instructions are typically provided when the user receives the device and supplies, but storage steps might also be relevant after each injection.
- Clean after use. Provide specific cleaning instructions for durable device components so that users do not damage the components with improper technique or cleaning agents.
- Storage location. Clearly indicate storage conditions, and seek ways to facilitate proper storage in common scenarios. For example, consider providing users with a carrying case designed to contain the device and related supplies for a week (or a day pack, if the drug is taken multiple times daily.)
CONCLUSION
Like any other medical device, developing wearable injectors that are safe, effective, and appealing requires a thorough user-centred design and human factors engineering process. Thinking through the most common use steps and potential use-related issues is a foundational exercise that should be informed by research and continuously repeated and refined. In doing so, companies designing and developing wearable injectors can build on their understanding of the user’s workflow through requirements definition, design, risk analysis, formative usability testing, instruction and training development, and ultimately human factors validation testing scenarios.

