Citation: Philippson J, Kemp T, “The Interface Between Prefilled Syringe and Autoinjector– A Development Framework”. ONdrugDelivery Magazine, Issue 101 (Oct 2019), pp 14-17.
Jeffrey Philippson and Tom Kemp outline the challenges for autoinjector developers at the interface between the device and the prefilled syringe – and describe a framework to formalise analysis of the interface.
The prefilled syringe (PFS) has widely published benefits over the traditional syringe and vial,1 leading to an increasing share of the drug delivery market in recent years.2 The addition of an autoinjector to deliver a PFS can enhance safety and the patient experience,3 opening up new opportunities for home- and self-administration.4
“Formed glass barrel meets precision-moulded parts, stopper glide force meets dynamic device spring-load, and syringe robustness meets plunger impact load.”
The typical starting point for development of an autoinjector solution, whether a novel development or application of a market-ready device, is an existing PFS used in clinical trials – but this leaves little opportunity for syringe selection or modification. Many of the technical challenges facing device developers stem from dysfunction at the interface between the device and the primary container and nowhere is this more evident than at the interface between PFS and autoinjector: formed glass barrel meets precision-moulded parts, stopper glide force meets dynamic device spring-load, and syringe robustness meets plunger impact load.
This article outlines the key features of the interface between device and syringe, addressing the functional dependencies and the challenges that can arise. A framework is then described to formalise the analysis of these features through the introduction of an interface specification that brings together overlapping requirements of the two subsystems to ensure compatibility and drive the development of test methods that directly address critical interdependencies.
Finally, we extend the interface concept beyond purely functional considerations to include organisational challenges – dysfunction at the interfaces within and between organisations involved in selection and development of autoinjectors can mirror challenges within the device. The benefits of getting it right are far-reaching and include rapid and effective evaluation of new PFS-autoinjector pairings, improved device performance and reliability, and accelerated development timelines.
“The choice of mounting location and geometry defines many aspects of the final assembled device, including critical requirements such as injection depth.”
CRITICAL DIMENSIONS AT THE GEOMETRIC INTERFACE
The most obvious point of contact at the interface is the axial mounting of the PFS within the device. Early autoinjectors mounted the PFS at the flange, which has the natural perpendicular geometry to resolve the axial load during syringe emptying. Unfortunately, the geometry that makes it so apparently suitable for mounting also makes it a key weak point that is vulnerable to fracture under load. Unacceptably high rates of breakage on firing were attributed to stresses on the flange, leading to recalls in extreme cases.5 Something had to change.
Most autoinjectors currently on the market mount the PFS at the shoulder, where the needle meets the glass body, which is the only other suitable surface. This change was adopted to reduce stress on the flange.6 However, the impacts of that decision went far beyond the initial drivers that motivated it.
The choice of mounting location and geometry defines many aspects of the final assembled device, including critical requirements such as injection depth. The syringe shoulder is created by forming semi-molten glass over a tungsten pin, in a process that inevitably results in a variable geometry, although this can be mitigated by post-forming inspection. To make matters worse, although all manufacturers comply with the ISO-specified dimensions, varying processes can still result in different nominal geometries.
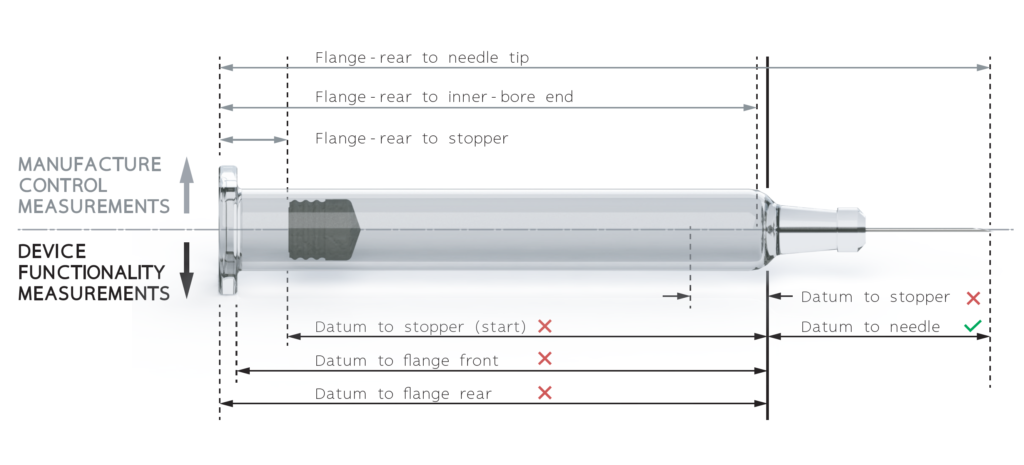
Figure 1: Diagram showing dimensions for manufacturing control and those critical to device functionality. A red cross indicates dimension typically not provided; a green tick indicates dimension typically provided. The details can vary from one manufacturer to another. The datum is the point on the syringe shoulder in contact with the device.
Needle placement during manufacture and stoppering during fill and finish are usually referenced to the flange, resulting in unpredictable offsets that appear as variability in critical dimensions such as the shoulder-to-needle-tip distance. Figure 1 shows the critical measurements from the points of view of manufacturing and device functionality.
“In the new world of biologics, with higher spring loads required to accommodate increased volumes and viscosities, we as an industry must remain vigilant.”
The story of in-use syringe breakages may have further twists in store. In the new world of biologics, with higher spring loads required to accommodate increased volumes and viscosities, we as an industry must remain vigilant and ensure the limits to syringe robustness are well understood.
FORCE BALANCE AT THE INTERFACE
In addition to the geometric interface, the balance of forces between PFS and autoinjector is also critical to device performance (Figure 2). The spring rate and precompression must be sufficient to overcome the stopper break-loose force to initiate syringe emptying, exceed the glide force to prevent stalling and achieve the required injection time. At the upper limit, spring load is limited by available space and the maximum impact force to avoid damage to the syringe and material creep in moulded parts. Although long springs with a low spring rate can seem like an ideal solution, with almost constant spring load, equilibrium spring length is limited by the challenge posed by compressing very long springs as part of an automated assembly process.
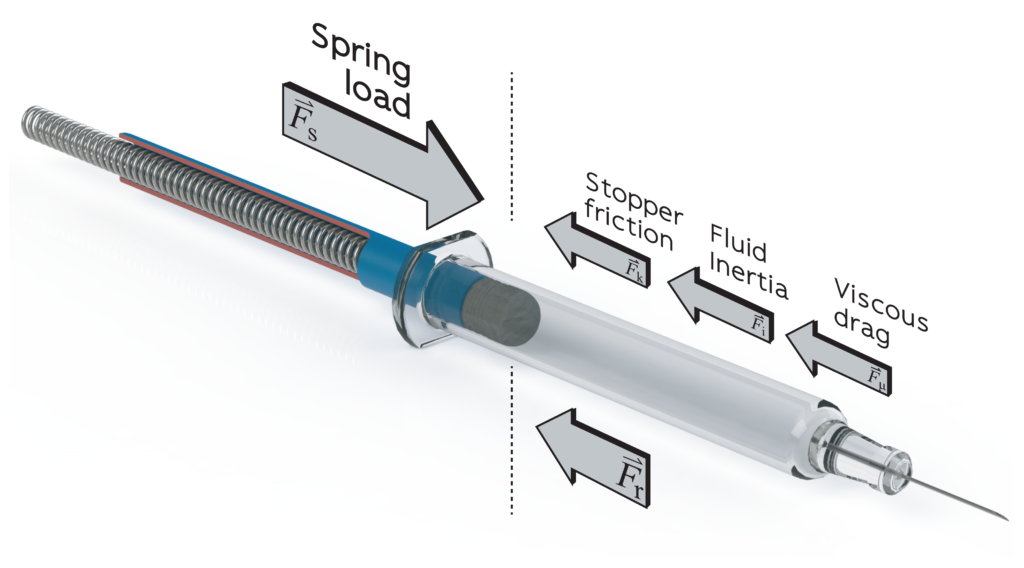
Figure 2: A force diagram showing a prefilled syringe being emptied under the device spring load, showing the spring load (Fs), stopper friction (Fk), fluid inertia (Fi), viscous drag (Fμ) and the resultant force (Fr). Arrows not to scale.
The standard force characteristics measured by manufacturers and users of PFSs in characterisation and quality control studies include the break-loose force and glide force at constant velocity. Glide force is generally reported as a mean value at a given velocity, whereas design for a specified injection time must take account of the dynamic force profile of the combined drive system and load. Consider that the spring load is constantly decreasing as the spring expands, while the stopper friction varies with both velocity and lubrication. Siliconisation reduces stopper friction but lubricant mobility during stopper travel can add further variability.7
A critical characteristic that can be extremely challenging to quantify is the threshold force for impact breakage. On release, the plunger accelerates towards the stopper under the applied spring load, generating a large transient force on impact. Designers attempt to minimise the initial gap between the plunger and stopper but the gap must be sufficient to avoid contact before firing, accounting for dimensional tolerances. Impact forces can be modelled or measured relatively easily but the corresponding threshold for syringe breakage is more of a challenge.
“A critical task when integrating a PFS with an
autoinjector is a detailed characterisation of the primary container, matching up measured characteristics of the syringe with their counterparts in the device.”
Simply replicating the conditions inside an autoinjector is not expected to yield observed breakages for any reasonable test sample size. A nonparametric tolerance limit can be placed on breakage rate based on observed incidence of non-breakages but demonstrating an acceptably low breakage rate would again require unrealistic sample sizes.
For example, demonstrating a breakage rate below one part-per-million with 95% confidence would require observation of 2,995,731 firings with no breakages. Alternatively, a parametric approach can be used, measuring the force at which breakage does occur by using much higher loads than those applied during device firing. In principle, the distribution of breakage forces can be compared with the measured device impact force distribution, with the overlap integral between the two representing the expected breakage rate and the difference between the tolerance limits on the two distributions representing a conservative safety margin.
There are limitations to this approach: a test apparatus that can reliably achieve syringe breakage cannot be fully representative of device spring load, materials or mechanical construction. The choices of spring rate and precompression influence the observed breakage force, necessitating a stepped approach with multiple impacts at increasing forces to produce a conservative estimate of the threshold breakage force. Statistical analysis of the results must be undertaken with care; breakage processes tend to be non-normal, with long high tails that can lead to misinterpretation of results.
Cap removal force is an important characteristic of autoinjectors and, for drugs indicated for acute conditions, can be an essential performance requirement. Although a modest additional friction component is added by the device, cap removal force is dominated by the force to remove the needle shield. Such high forces are required to accommodate the variable seating geometry, while maintaining container closure integrity. The standard claimed upper limit on needle shield removal force is 35 N, constraining the design window for cap removal force and leading to usability problems.3 Although most fall well below this limit, forces over 25 N are not uncommon, particularly at low temperature. Many syringe manufacturers are now filing IP for needle shield removal tools for manual administration8,9 and some device developers are adding mechanisms to assist with cap removal.10
A FRAMEWORK FOR ADDRESSING OVERLAPPING REQUIREMENTS AT THE INTERFACE
A critical task when integrating a PFS with an autoinjector is a detailed characterisation of the primary container, matching up measured characteristics of the syringe with their counterparts in the device. A useful framework for this is the concept of an interface specification, with requirements defined in terms of functionality and referenced to both the design input requirements of the device and a specification for the PFS.
A clearly defined interface specification can be used to define a matched set of characterisation tests to assess compatibility between autoinjector and PFS. The development of the specification and the associated tests is a crucial learning step that should be integral to the device development process. It further comes into its own when the inevitable question is asked: “Can the device deliver a different PFS?” Whether it is a new drug product, a new syringe or both, the interface specification provides a clear process and unambiguous answers to critical questions of compatibility.
In an ideal world, the syringe requirements would simply be taken from the manufacturer drawings and other specifications. However, life is rarely so simple. Dimensions specified by manufacturers relate to the manufacturing process rather than to optimal seating within an autoinjector, having been developed for manual administration. The mass-produced glass prefillable syringe has existed for 65 years11 and is highly optimised as a low-cost, sterile, high-barrier primary container and drug delivery system, manufactured in enormous volumes.
THE INTERFACE WITHIN AND BETWEEN ORGANISATIONS
Beyond purely functional considerations, challenges extend to the organisational interface between primary container and medical device teams. Pharmaceutical companies have long had dedicated teams focused on evaluation and selection of primary containers, including syringes, which play critical roles protecting the drug product, maintaining sterility and achieving the required shelf-life. More recently, dedicated medical device teams have appeared, focused on evaluation and selection of devices such as autoinjectors.
The relatively recent appearance and generally low profile of medical device teams can lead to a disconnect between them and the wider organisation, with limited communication and a lack of joined-up decision making. This mirrors the siloed mentality that has traditionally existed between the chemistry, manufacturing and control (CMC) and research and development (R&D) divisions.
Overcoming this divide has the potential to pay significant dividends through sharing of expertise, strategic decision making and early consideration of opportunities for drug delivery devices when selecting a primary container for clinical trials. Typically, primary container selection is locked down long before any consideration is given to medical device options and there is a natural reluctance to change the container selection when proceeding to device selection or development.
The concept of the organisational interface can be extended to address the interface between the business models of syringe manufacturers and pharma companies. Syringe procurement by pharma companies considers a wide range of characteristics but historically there has been no reason to include the external dimensions in this assessment – and the manufacturing process for production of glass syringes reflects this. Some manufacturers are willing to provide glass syringes with tighter dimensional tolerances through post-selection using automated vision systems but this approach has not been widely adopted due to increased costs. With rapid growth in the autoinjector market, optimisation through better alignment across the supply chain has the potential to benefit all parties.
For many reasons, syringe manufactures have been slow to adopt polymer syringes: the excellent barrier properties of glass; sensible risk aversion, a “go with what you know” attitude; and some inevitable inertia. Japan is a notable exception, having fully transitioned to moulded polymer syringes, cartridges and vials, partly due to the risk of glass breakage during earthquakes.
Polymer syringes would certainly address concerns around dimensional tolerances and the Japanese example demonstrates the effective and scalable nature of this solution. Market shifts point to a steady increase in the use of polymer syringes and that is one way to address complexities at the interface with drug delivery devices.
CONCLUSION
Dysfunction at the interface between prefilled syringe and autoinjector? With a clear view of the critical inter-dependencies and a well-characterised syringe, it need not come to that. We have a vision of prefilled syringe and autoinjector working together in harmony and, with good communication within and between partner organisations, we as an industry can make it happen.
REFERENCES
- Makwana S et al, “Prefilled syringes: An innovation in parenteral packaging”. Int J Pharm Investig, 2011, Vol 1(4), pp 200–206.
- Mordor Intelligence, “Syringe Market – Growth, Trends, and Forecast (2019–2024)”. April 2019.
- Weinhold T et al, “Improving the safety of disposable autoinjection devices: a systematic review of use errors”. AAPS Open, 2018, Vol 4(7), pp 1–14.
- Bernstein D et al, “Usability of mepolizumab single-use prefilled autoinjector for patient self-administration”. J Asthma, 2019, Vol 56, pp 1532–4303.
- DeGrazio F, Paskiet D, “Glass breakage, delamination and compatibility with biologics have boosted interest in novel materials in pharma packaging”. ContractPharma.com, 2012.
- Nemera.net, “Safelia® 1 ml and 2.25 ml Autoinjectors Designed to be Patient Friendly”. Product leaflet, 2014.
- Liebmann-Vinson A, “Physics of Friction Applied to Medical Devices”. Microstructure and Microtribology of Polymer Surfaces, 1999, Chapter 30, pp 474–494.
- Allen T, “Two piece needle shield puller”. US Patent Application, 2017, US20170274151A1.
- Denzer M et al, “Autoinjector apparatus”. WIPO Patent Application, 2012, WO2012145685A1.
- Julian J et al, “Removal of needle shields from syringes and automatic injection devices”. US Patent, 2014, US8708968B2.
- Wright J, Soukiassian H, “Prefillable syringe technology – BD Neopak – Delivering the Next Generation in Glass Prefillable Syringes”. Drug Dev Del, 2014, Vol 14(1), pp 55–61.

