Citation: Augustyn S, “How to Design a Body-Worn Injector”. ONdrugDelivery Magazine, Issue 105 (Feb 2020), pp 61-64.
Body-worn injectors require you to understand your user, your drug and your technology – and there are numerous pitfalls for the unwary. Stephen Augustyn shares some insights.
“A body-worn injector intended to treat a patient recovering from surgery will have a very different set of requirements from one intended to help a patient manage a chronic condition.”
Body-worn injectors are tricky little beasts – they aim to combine the convenience of an autoinjector with the precision of an infusion pump but aren’t quite either of these devices. There is a wealth of devices and technologies on the market – or about to reach the market – all vying for your attention. But how can you make sure you’re choosing the correct product or making the right design decisions?
It really comes down to three key points – understand your user, understand your drug and understand your technology (Figure 1). If you have secure knowledge of these three areas, your chance of creating – or selecting – the right device increases enormously. Team Consulting has spent the last six years designing and identifying the right body-worn injectors for clients and contributing to the new ISO standard to help regulate these products. This article offers a distillation of some of the key things that we have learned.

Figure 1: Understand your user, your drug and your technology.
There are some excellent products in this space but there is no single device that will be the optimum choice in all applications. A body-worn injector intended to treat a patient recovering from surgery will have a very different set of requirements from one intended to help a patient manage a chronic condition.
UNDERSTANDING YOUR USER
There are many reasons why you may wish to use a body-worn injector over an autoinjector – you may be delivering more drug than an autoinjector can comfortably hold, you may wish to spend longer delivering the drug or you may even want to delay, or modulate, the drug delivery. However, the single biggest driver for body-worn injectors has been the requirement to deliver larger drug payloads. Most autoinjectors on the market have a prefilled syringe (PFS) at their heart – a rigid glass or polymer syringe, with a sterile needle, storing 1-2.25 mL of drug product.
“The design of the patch and careful consideration of the removing features is also critical.”
The principal difference between an autoinjector and an on-body injector is that the patient holds the autoinjector throughout the delivery sequence whereas, with a body-worn injector, the user attaches the device, begins the delivery and removes the device once delivery is complete. This aspect of on-body injectors should result in a more comfortable and consistent injection experience for the patient but it does require a more sophisticated and expensive device.
ATTACHING AND REMOVING THE INJECTOR
Precisely how the injector will attach to the body should be a key consideration for the designer. If the device is a single-use disposable product, adhesive would be the obvious choice but even that solution requires some deeper consideration. The requirements for an adhesive that only needs to work for 10 minutes whilst a patient is stationary are very different from the adhesive that needs to work on an active user and stay in place for hours.
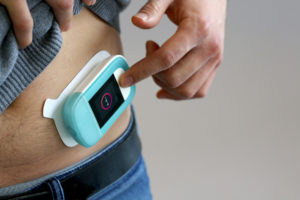
Figure 2: How the injector will attach to the body is a key consideration.
The profile of the patient will also make a big difference – young children and the elderly can have very delicate skin, and some conditions or drugs may make the skin more susceptible to damage and irritation. The design of the patch (Figure 2) and careful consideration of the removing features is also critical – the patch should allow the user to stabilise the skin around the patch, get a secure grip on the patch and remove it with a gentle peeling action. 3M has a useful “find my adhesive” facility on its website that can help you identify a suitable material but be prepared to do some testing and evaluation work.
If you have a device with an integral needle or cannula, you will have no option but to affix the product directly to the skin – but there is the option of remote delivery. This is the model used by most continuous subcutaneous insulin infusion (CSII) pumps and can be practical for disposable pumps as well. Patients with chronic conditions may present problems when asked to adhere a pump to the same area of the body day after day, so separating the pump from the infusion site may offer a real advantage.
USER INTERFACE
On-body injectors will often feature electronics as part of the delivery system and this will then come with the temptation to give ever more feedback to the user, add smartphone integration and offer “helpful” advice on use of the product. This is a huge area to explore, and beyond the scope of this article, but every new feature must justify its place on the product and what may seem like an incredibly helpful and smart feature in the design office may be very confusing to patients. It’s worth dedicating significant time and effort to investigating and testing products with target users as it will reveal challenges you didn’t see coming. Get your product concepts in front of representative users as early as possible and let your decision making be based on evidence.
CO-MORBIDITIES AND COMPLEX TREATMENTS
“The intellectual property landscape around these drive and sensing technologies is extremely crowded and identifying freedom to operate can be a significant challenge.”
It is increasingly the case that users present to their healthcare practitioners with complex conditions – e.g. diabetes, high blood pressure and diabetic neuropathy. These are precisely the patients who could most benefit from a body-worn injector and their needs will have to be considered if they are your target group. If the device is not sold prefilled and the user needs to transfer or reconstitute a drug, how is the patient going to manage this process?
The ISO Committee ISO TC84 examined the challenges that visually impaired users have with injectors when it created ISO 11608-7:2016. The design guidance in ISO 11608-7 has many useful recommendations that may assist designers developing new on-body devices.
UNDERSTANDING YOUR DRUG
One of the key questions that can impact the design of a body-worn injector is whether the device will come prefilled or not. If the device will be prefilled, there will be a fundamental impact on the design of the primary container, the environmental storage of the drug/device combination and the stability studies undertaken. Storing the injector below room temperature will have an impact on the materials and power sources in your device. Even a few degrees’ change can make plastic materials more brittle and less flexible. This could mean that the critical fluid seal on your device no longer works as it did during your development testing, your injection forces become larger or fluid lines don’t flex as they should.
The effect of temperature on drug behaviour should also be examined in detail and testing should reflect real-world use of the product. If the drug and device will be stored at low temperatures and then brought up to room temperature for delivery, the test programme should reflect this. A lower temperature drug may have a much higher viscosity which would require more force to be delivered than the mechanism can produce. Similarly, testing with a drug or mimic at a higher temperature than intended may result in a false positive in the testing programme – putting the health of patients at risk.
Aiming to deliver a larger drug payload or a high-viscosity drug is one of the benefits of using an on-body injector. The challenge comes with using a suitable model fluid during development testing. Some of the drugs destined for these devices are extremely expensive or highly toxic. But if you leave your device testing with the real drug – or a very accurate mimic – until the last moment, you can guarantee that it will have some surprises for you.
BACK TO PRIMARY SCHOOL
Primary container selection will be at the heart of the device design. There are hundreds of filling lines available in the world’s major economic areas that have been configured to fill standard cartridges and prefilled syringes – and if your container will fit on this line, you have an immediate commercial advantage. There may be a compelling reason for adopting a novel primary container but if your pharma clients are very reluctant to re-run stability studies in new containers, you will need to apply careful consideration as to how you get the drug from a vial or cartridge into your on-body injector.
Experience from running dozens of verification studies on different parenteral products suggests that ageing products and introducing a live drug for the first time always have an impact on device performance. So, start testing with either the active drug or a highly representative placebo as early as you can in the development as you don’t want to be making fundamental discoveries on device behaviour when you get to your design verification programme.
UNDERSTANDING YOUR TECHNOLOGY
In addition to your primary container, you will need to provide a source of power to deliver the drug. On most autoinjectors this is performed using a compressed spring and there is no reason why a spring can’t be used on a body-worn injector. Compression springs are cheap, accurate, reliable and easily made to your specification. They are also large, need to be stored in a compressed (highly loaded) form, can’t be controlled after release and lose power as they expand.
If you have a highly variable injection force (for example, a high break-free force and a low glide force) then springs may not be ideal. Also, if you need a device with a slow infusion of drug, you’ll need to control this action through flow restrictors in the fluid path and this can lead to variable behaviour as the drug viscosity changes or as the manufacturing tolerances change.
Many of the restrictions of using springs don’t matter for autoinjectors where you are just trying to deliver the contents of the primary container as quickly as possible. However, in body-worn injectors, springs may be too much of a compromise. If you need to provide active control of the delivery of the drug, you are almost certainly looking at an electronic drive system with rate control. This can be done with no feedback (where you may just be driving a pump or plunger at a defined rate) or you may use active feedback to precisely control the delivery rate.

Figure 3: Displacement pump technology is at the heart of Sensile’s range of pumps.
Active feedback, measuring displacement or fluid flow, offers the best control of delivery rate and it will also provide a mechanism to detect occlusions or alert the user to any errors in the device. This would necessitate having an electronic drive system which could be a displacement pump like the SenseCore pump at the heart of Gerresheimer subsidiary Sensile Medical’s range of pumps (Figure 3) or a plunger-based system as used in West Pharmaceutical Services’ SmartDose device. The SMT-101 pump from United Therapeutics subsidiary SteadyMed Therapeutics, uses a novel expanding battery design and there are many other competing technologies that different manufacturers are promoting. The intellectual property landscape around these drive and sensing technologies is extremely crowded and identifying freedom to operate can be a significant challenge.
The use of electronics does come with one significant headache that pharma companies aren’t used to – supply management for electronic components. The electronics industry works on a very short lifecycle, driven largely by the rise in mobile consumer technology. Resolving the tension between having a stable design (as expected in a lot of pharma applications) and a key piece of technology in your product, such as a sensor or a drive system, that will be going through generational changes over the life of your product – which is likely to take years to get to market – presents real challenges. Speaking directly to the supplier of these parts and committing to holding stock of components may be the only real strategy for a device builder.
INJECTING OR INFUSING?
Selection of a drive technology will be influenced by your intention to deliver an injection or an infusion. The difference is that an injection is based on delivering a volume of drug in a manner tolerable to the patient – and an infusion is based on delivering drug at a controlled rate to create a pharmacokinetic effect. This is the critical difference that has been identified in the draft of ISO 11608-6, the new standard for on-body delivery systems.
For example, CSII pumps must deliver basal and bolus quantities of drug at a precisely controlled rate and, for this reason, their performance requirements are best described by IEC 60601-2-24. This also applies to the Insulet (Acton, MA, US) Omnipod, which can superficially appear to be a large-volume injector but is in fact an infusion pump. Whilst a product that acts as an infuser or an injector will face many of the same challenges, it is vital that the device manufacturer recognises what is important to them. This required performance will have a dramatic effect on the architecture of their device and the verification that they will have to evidence.
GETTING THE ‘MEOST’ OUT OF YOUR TEST PROGRAMME
One of the key approaches to mitigate hazards from unknown drug behaviour or technical design risks is to use multiple environment over stress testing (MEOST) or highly accelerated life testing (HALT). Both methodologies will help to stress your product by pushing the testing outside the specification range for the device. This testing should not be part of your verification programme but part of an engineering test programme or a small-scale pre-design verification test (DVT) study.
This approach to testing will help you to be confident that the design space you have defined is well within the capabilities of your device. If you only ever test to your device specification limits, you’ll never know if a subtle change in component tolerances or manufacturing process may result in a huge spike in device failures.
You can also use analytical modelling to identify vulnerabilities in the design by running multiple simulations of the way the product will operate to help understand your design space. These analytical modelling approaches do not need to be limited to complex 3D simulations that rely on heavy computing power – something as simple as a Monte Carlo analysis of tolerance stacks or activation loads may save a lot of time and samples in the test lab.
CONCLUSION
On-body injectors offer some significant benefits for patient care, including management of chronic symptoms and less time in hospital. The key to delivering a successful product is all about putting the patient experience at the centre of the design, building the device to deliver that experience and knowing exactly how your drug and device technology will behave. Whilst, superficially, these products can look a lot like infusion pumps or autoinjectors, they come with their own challenges – and having a rigorous test and development programme in place is your very best chance of creating a robust product.

