Citation: King J, Huddleston M, “Patient Tolerability With High-Viscosity, Large-Volume Subcutaneous Infusions”. ONdrugDelivery Magazine, Issue 97 (May 2019), pp 40-43.
Jennifer King and Matthew J Huddleston discuss the advantages of subcutaneous drug delivery and how these have yet to be fully harnessed in currently marketed therapeutics.
Prepared by ONdrugDelivery for and on behalf of Enable Injections based on two blog articles, “Advantages of Subcutaneous Drug Delivery” and “Patient Tolerability with High-Viscosity, Large-Volume Subcutaneous Infusions”, originally published earlier this year on the Enable web page.
ADVANTAGES OF SUBCUTANEOUS DELIVERY
Recent innovations have led to the development of numerous novel small-molecule and biologic formulations that require parenteral administration, mainly intravenous (IV), subcutaneous (SC) or intramuscular (IM). Concurrently, innovative drug delivery systems, such as infusion pumps, autoinjectors and wearable infusers, have been developed to facilitate patient access to parenteral therapies.
The number of new product approvals of parenterals has risen in recent years accordingly (see Figure 1), in particular of products for SC delivery. There were 95 biologic therapies for SC administration approved by the US FDA in 2017 alone and approximately 240 SC biologics are either in development or have been submitted for approval by the FDA.1
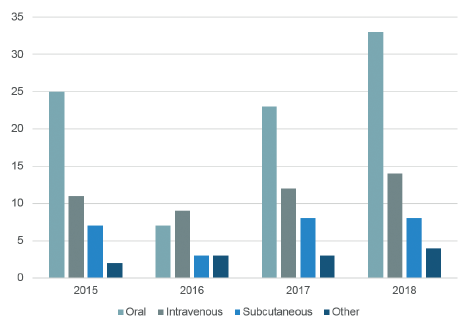
Figure 1: 2015-2018 US FDA novel drug approvals by year and route of administration.
IV administration requires a medical facility with trained medical professionals, travel to and from which is inconvenient, often prohibitively so, for many patients. IV administration also typically requires a trained medical professional to set-up an IV infusion set and determine appropriate dosing/infusion rate and time. In addition to the risk of infection at the injection site, IV administration is also associated with the risk of systemic infection, potential exposure to IV particulate matter, and exposure to hospital-acquired infections.
“What is it like to inject 5, 10, 15 mL and greater volumes of a high viscosity biotherapeutic into SC tissue at high flowrates? More importantly, can the patient continue to tolerate this approach?”
For IV administration, additional time is required before, during, and after the actual infusion for the preparation, infusion duration, and observation post-infusion. Because of the extended time, supplies, facility, trained staff, and equipment required for administration, IV therapeutics are generally more expensive compared with other methods of administration.2
SC administration allows therapeutics to be self-administered by patients or healthcare providers using a variety of different delivery systems. Because SC administration facilitates patient self-administration in home or outpatient clinical environments, it reduces medical facility fixed costs. In a 2015 Belgian meta-analysis evaluating socio-economic impact of SC versus IV administration of trastuzumab in HER2 positive metastatic breast cancer, it was estimated that SC administration could contribute to a cost saving of €758-2576 (£653-2376) per annual course. SC administration was also reported to enable a threefold reduction in the total preparation and administration compared with IV administration.3
SC administration provides flexibility in the anatomical infusion site, and options include the stomach, thighs, and backs of the arms. SC infusion systems can be designed with smaller needle sizes, which may decrease pain during infusion.
While the risk of infusion site infection still exists, SC infusion site infections are generally limited to cellulitis and very rarely progress to systemic infections. Further, administration at home reduces the risk of exposure to hospital-acquired infections.
In a 2015 meta-analysis, a literature search was conducted to identify clinical studies published from 1980 to February 2015 that included comparison of IV, IM, and SC administration in order to determine the advantages and disadvantages of each administration route and investigate patient preference. In studies comparing SC to IV administration, more patients reported a preference for SC administration (88.9%) than IV (9.6%).4 An international, randomised, two-cohort study (PrefHer) reported similar results, in which 92% of patients stated they preferred SC administration of trastuzumab versus 8% for IV.3
In addition, a systematic review of randomised, controlled trials and/or crossover studies investigating patient preference reported that patients preferred SC versus IV administration in four of six trials.5 In addition, a non-interventional time and motion study was conducted in 2016 in eight countries to determine time savings with rituximab SC injection compared with IV infusion. The study determined that patient SC self-administration at home decreased treatment time.6
THE NEED FOR SOMETHING DIFFERENT
Traditional methods of SC drug delivery include autoinjectors and infusion pumps. These delivery regimens have traditionally been limited by the volume which can be delivered (<1-2 mL), injection site degradation of the therapeutic (absorption), and dose range. But with recent advances in formulation and delivery technologies, SC is increasingly becoming a viable means of administering a wide variety of therapeutics.
Patient experience is becoming more important, as it relates to overall drug efficacy and safety. The introduction of biotherapeutics, which require higher concentrations of active pharmaceutical ingredient to meet efficacy requirements, has not only challenged the previously established limits of volume, viscosity, and flowrate, but the devices used to deliver them. Thus, while the trend for SC administration is moving to large-volume injections of high-viscosity drugs, there are many questions that are not well understood. For example, what is it like to inject 5, 10, 15 mL and greater volumes of a high-viscosity biotherapeutic into SC tissue at high flowrates? More importantly, can the patient continue to tolerate this approach?
“Key device design characteristics that may influence patient experience and tolerability during SC infusion of high-viscosity, large-volume therapeutics include flow rate, skin/needle interface, and needle size.”
Many peer-reviewed studies7,8,9,10 have focused on administration volumes of up to 3 mL, flowrates of up to 10 mL per minute, and viscosities of up to 50 cP. These studies have been singularly focused on one or two performance attributes due to limitations with the devices used for delivery. Data is needed on patient tolerability with the combination of large volumes, various flow rates, and higher viscosities associated with SC infusion.
Devices need to adapt to this new class of drugs. The best design will incorporate a unique set of device attributes and requirements, as well as anticipate key drug and patient variables to produce a safe and efficacious delivery, while maximising a positive patient experience.
DEVICE VARIABLES & CONSIDERATIONS
Key device design characteristics that may influence patient experience and tolerability during SC infusion of high-viscosity, large-volume therapeutics include flow rate, skin/needle interface, and needle size. These attributes are discussed further below with respect to patient impact. It is also likely that these variables are interdependent.
Flow Rate
Previous studies9 have focused on high-speed injection (autoinjectors) or constant flowrate delivery (infusion pumps). The value proposition has been to minimise injection time or allow for a fixed injection time, both of which could be desirable if, for example, the user needs to hold an injection device against their skin or be tethered to an infusion pump.
Infusion time may become less relevant if the user is uninhibited during daily activities while receiving therapy, such as using an on-body delivery device, and a slower flowrate (and the associated longer injection time) might well be preferred by the patient and improve tolerability of large volumes. It is hypothesised that slower flowrates could lead to a lower incidence of site reactions and injection pain.
Skin / Needle Interface (Tissue Tent)
In general, previous studies10,11,12 have investigated SC injection by needle and syringe, or using infusion sets and butterfly needles. In either case, the user has the ability to control the depth of the needle by either pinching or stretching the tissue at the injection site prior to needle insertion.
This pinching or stretching, known as the tissue tent, may also provide a secondary benefit by applying a small amount of pressure at the injection site, which may have several advantages, including reducing needle insertion pain by disrupting the tissue at the injection site, similar to a nurse pressing on the injection site prior to needle insertion.
It is also hypothesised that pressure at the injection site could encourage a deeper deposit of drug into the subcutaneous space, especially in cases of large delivery volume. It could also potentially prevent leakage and backflow, similar to a nurse applying pressure to the injection site at the completion of delivery.
Whilst manually inserted needles require manual pinching / stretching, devices can incorporate a mechanism to stretch the tissue at the injection site automatically, ensuring the needle inserts to the correct depth, that drug is delivered into the appropriate anatomical space and conferring any additional advantages relating to pain and drug deposition.
Needle Size
Previous studies with needles and syringes, or infusion sets, typically involve needles ranging from 31-26 gauge, with the majority being at the larger end of the range. A few publications have theorised that smaller-gauge needles introduce greater discomfort due to increased fluid velocity at a constant flowrate.13,14 However, this is unlikely with low pressure delivery. It is likely that flowrate in conjunction with needle size could be important, with a smaller needle size being preferred. In general, smaller needle sizes produce less injection site pain11,12 with less leakage and backflow.
DRUG VARIABLES AND CONSIDERATIONS
Characteristics of the drug that impact patient tolerability of high-viscosity, large-volume SC infusion must be considered.
Volume and Viscosity
Typically, pharmaceutical companies have had the mindset to pursue high-concentration (high-viscosity) and low-volume formulations based on limitations with previously available delivery systems. But studies suggest that injection site pressure is less affected by volume and more dependent on viscosity.10,12 Pharma and biopharmaceutical companies could instead take advantage of the flexibility in the drug concentration and delivery volume that high-volume injectors allow, to open up new possibilities in formulation development for SC administration.
Other Drug-Specific Attributes
Other drug-specific variables which affect patient tolerability may include pH, osmolality, excipients, and temperature. Much is known about patient tolerability with low-volume SC injections,15 but what is the pain tolerance and how is it affected by these drug-specific attributes of the therapeutic?
PATIENT VARIABLES AND CONSIDERATIONS
Injection Pressure and Backpressure
Previous studies have investigated tissue backpressure as a function of flowrate, viscosity, and delivered volume.8,16 However, these studies were executed with constant flowrate pumps. Studies of a system that adjusts the flowrate of the drug based on the backpressure being created within the injection site could explore potential advantages in terms of patient preference and tolerability compared with constant flowrate pumps.
Patient Characteristics
The abdomen is a popular location for SC injections, due to its easy access and amount of available space. Additional SC injection sites, including the inner thigh and back of the arm, could also be considered, although the suitability of these sites for larger infusion volumes is not established. The impact of other patient demographics, such as body mass index (BMI) and skin integrity/type, on infusion and patient tolerability should also be evaluated.
REFERENCES
- “Subcutaneous Biologics, Technologies and Drug Delivery Systems (2nd Edition), 2018 – 2030”. Research Report, Roots Analysis, 2018.
- Schmier J et al, “Costs of Providing Infusion Therapy for Rheumatoid Arthritis in a Hospital-based Infusion Center Setting”. Clin Ther, 2017, Vol39(8), pp 1600-1617.
- Papadmitriou K et al, “The Socio-economical Impact of Intravenous (IV) Versus Subcutaneous (SC) Administration of Trastuzumab: Future Prospectives”. Facts Views Vis Obgyn, 2015, Vol 7(3), pp 176-180.
- Jing-fen J et al, “The Optimal Choice of Medication Administration Route regarding Intravenous, Intramuscular, and Subcutaneous Injection”. Patient Prefer Adherence, 2015, Vol 9, pp 923-942.
- Stoner K, Harder H, Fallowfield L, Jenkins V, “Intravenous versus Subcutaneous Drug Administration. Which Do Patients Prefer? A Systematic Review”. Patient, Jul 2014 (epub ahead of print).
- De Cock E et al, “Time Savings with Rituximab Subcutaneous Injection versus Rituximab Intravenous Infusion: A Time and Motion Study in Eight Countries”. PLoS ONE, 2016, Vol 11(6), e0157957.
- Berteau C et al, “Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance”. Med Devices, 2015, Vol 8, pp 473-484.
- Zijlstra E et al, “Impact of Injection Speed, Volume, and Site on Pain Sensation”. J Diabetes Sci Technol, 2018, Vol 12(1), pp 163-168.
- Praestmark K et al, “Injection Technique and Pen Needle DesignAffect Leakage from Skin After Subcutaneous Injections”. J Diabetes Sci Technol, 2016, Vol 10(4), pp 914-922.
- Patte C et al, “Effect of infusion rate and indwelling time on tissue resistance pressure in small-volume subcutaneous infusion like in continuous subcutaneous insulin infusion”. Diabetes Technol & Ther, 2013, Vol 15(4), pp 289-294.
- Doughty D et al, “Understanding Subcutaneous Tissue Pressure for Engineering Injection Devices for Large-Volume Protein Delivery”. J Pharm Sci, 2016, 105(7), pp 2105-2113.
- Allmendinger A et al, “Measuring tissue back-pressure–in vivo injection forces during subcutaneous injection”. Pharm Res, 2015, Vol 32(7), pp 2229-2240.
- Alam M et al, “Effect of Needle Size on Pain Perception in Patients Treated with Botulinum Toxin Type A Injections: A Randomized Clinical Trial”. JAMA Dermatol, 2015, Vol 151(11), pp 1194-1199.
- Pathak P et al, “Effect of Needle Gauge on Perception of Pain Intensity among Infants Receiving D.P.T. Vaccination”. Nursing Midwifery Res J, 2007, Vol 3(4), pp 172-178.
- Dias C et al, “Tolerability of High-Volume Subcutaneous Injections of a Viscous Placebo Buffer: A Randomized, Crossover Study in Healthy Subjects”. AAPS PharmSciTech, 2015, Vol 16(5), pp 1101-1107.
- Juul K et al, “Influence of hypodermic needle dimensions on subcutaneous injection delivery – a pig study of injection deposition evaluated by CT scanning, histology, and backflow”. Skin Res Technol, 2012, 18(4), pp 447-455.

