Citation: Arakawa S, Suzuki T, “OXYCAPT Multilayer Plastic Vial and Syringe”. ONdrugDelivery Magazine, Issue 105 (Feb 2020), pp 79-82.
Shota Arakawa and Tomohiro Suzuki, discuss the benefits of the OXYCAPT™ multilayer plastic vial and syringe for the rapidly growing field of biologics and regenerative medicines.
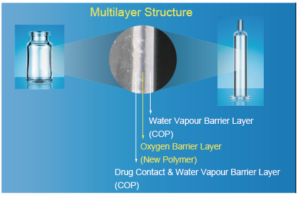
Figure 1: The multilayer structure of OXYCAPT.
There are some problems with existing glass and plastic vials and syringes. For example, glass suffers from breakage and delamination, whereas plastic lacks sufficient oxygen and ultraviolet light (UV) barrier properties. Especially with glass, the US FDA has pointed out these problems, which have led to more than 50 recall incidents. To address these problems with glass, several suppliers have launched alternative plastic vials and syringes – but in some cases the inadequate oxygen barrier has meant they have failed to meet customer demands. Given this situation, Mitsubishi Gas Chemical (MGC) has developed multilayer plastic vials and syringes with an excellent oxygen barrier, a high UV barrier, very low extractables and high breakage resistance, among other features.
“The oxygen barrier of the OXYCAPT-P vial is about 20 times better than that of a COP monolayer vial.”
The OXYCAPT™ vial and syringe consists of three layers (Figure 1). The inner and outer layer are made of cyclic olefin polymer (COP) – the most reliable polymer in the pharma industry. The middle layer is made of a novel polyester developed by MGC. The characteristics of COP give OXYCAPT™ a high water-vapour barrier, very low extractables, high pH stability, low protein adsorption, high breakage resistance, etc. The new polyester plays a role as an oxygen and UV barrier to address the weaknesses of COP alone.
EXCELLENT OXYGEN BARRIER
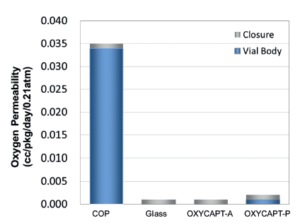
Figure 2: Oxygen permeability of the types of OXYCAPT.
There are two types of OXYCAPT™ multilayer plastic vial and syringe – OXYCAPT-A and OXYCAPT-P. OXYCAPT-A has achieved a glass-like oxygen barrier. According to some internal studies, OXYCAPT-A can keep a lower oxygen concentration in headspace than type 1 glass, thanks to its oxygen-absorbing function. Although there is no oxygen absorbing function in OXYCAPT-P, it has also achieved an excellent oxygen barrier. For example, the oxygen barrier of the OXYCAPT-P vial is about 20 times better than that of a COP monolayer vial (Figure 2). OXYCAPT-A is particularly suitable for oxygen-sensitive drugs and OXYCAPT-P is recommended for any drug.
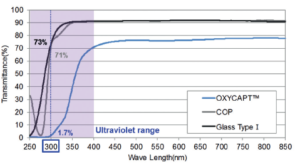
Figure 3: Ultraviolet light barrier of OXYCAPT™.
OXYCAPT™ also has UV barrier properties. For example, although about 70% of UV light of 300 nm transmits through glass and COP, only 1.7% UV light transmits through OXYCAPT™ (Figure 3). This feature also contributes to the stability of biologics.
When it comes to its water vapour barrier, OXYCAPT™ cannot reach the performance of glass. However, it is similar to COP which has been used for injectable drugs for a long time, and easily meets the requirements of a water vapour barrier stipulated in the ICH guideline.
LOW LEVELS OF EXTRACTABLES
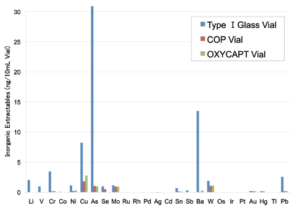
Figure 4: Graph of inorganic extractables.
OXYCAPT™ generates extremely low levels of extractables. For example, in one study that measured volatile, semi-volatile and non-volatile impurities from OXYCAPT™, water and four solutions (50% ethanol, NaCl, NaOH and H3PO4) were used and impurities were measured by gas chromatography-mass spectrometry (GC-MS) and liquid chromatography- UV spectroscopy-mass spectrometry (LC-UV-MS) after 70 days at 40°C. Compared with control, no impurities were detected in any of the OXYCAPT™ containers. A second study was conducted to measure inorganic extractables from OXYCAPT™. The level of extractables was similar to that from COP – which is well known as an extremely pure polymer – and less than that of type 1 glass (Figure 4).
The OXYCAPT™ syringe consists of tip-cap, barrel, PTFE-laminated stopper and plunger rod (Figure 5). Although the stoppers are coated with a very small amount of silicone oil, none is baked on the barrel. According to our internal studies using antibodies, we have found this feature noticeably reduces instances of protein aggregation compared with existing type 1 glass syringes.
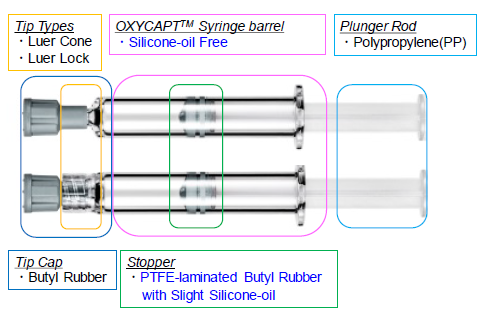
Figure 5: Components of the OXYCAPT syringe.
The OXYCAPT™ vial and syringe are produced by co-injection moulding technology. Although this technology has been applied to beverage bottles for many years, MGC is the first company to succeed in developing multilayer plastic syringes. We have also developed inspection methods for the oxygen barrier layer. All the containers are 100% inspected by state-of-the-art machinery.
FREE SAMPLES FOR INITIAL TESTING

Figure 6: The nest and tub.
MGC can offer bulk vials, ready-to-use (RTU) vials and RTU syringes. Regarding the RTU products, vials and syringes are provided in ISO-based nest and tub formats (Figure 6). The nest and tub are primarily sterilised by gamma ray. There are 2 mL, 6 mL, 10 mL and 20 mL variants for vials, and 1 mL long and 2.25 mL variants for syringes (Table 1). We are willing to provide samples for initial testing free of charge.
| Type | Volume | ISO | Parts | Option |
| Vial | 2 mL | ISO 8362-1 | Vial | Bulk or RTU |
| 6 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| 10 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| 20 mL | ISO 8362-1 | Vial | Bulk or RTU | |
| Syringe | 1 mL Long | ISO 11040-6 | Barrel, Tip Cap, Stopper, Plunger Rod |
RTU |
| 2.25 mL | ISO 11040-6 | Barrel, Tip Cap, Stopper, Plunger Rod |
RTU |
Table 1: Product portfolio.
Each polymer meets the requirements of USP661, USP87, USP88, EP, and has been filed in the US FDA’s drug master file (DMF). The vials and syringes are also compliant with each pharmacopoeia. The syringes are produced and controlled in accordance with ISO 13485.
The target therapeutic application for OXYCAPT™ is biologics. As the ICH guideline, “Stability of Biotechnological/ Biological Products Q5C” mentions, oxidation is one of the causes of protein instability. Some features of OXYCAPT™ – such as its high oxygen and UV barrier properties – contribute to the stability of biologics.
In addition, we believe OXYCAPT™ can be applied to epinephrine, as it is well known as an oxygen-sensitive drug. The breakage that can occur with glass syringes is not suitable for emergency drugs, so some suppliers have tried to develop new pen injectors made of plastic. Also, customers have started evaluating the OXYCAPT™ vial for their gene and cell therapy. Our ready-to-use vial sterilised by gamma is ideal for protein-based drugs.

Figure 7: Staked-needle syringe (under development).
Some customers have asked us to develop staked-needle multilayer plastic syringes, so we started tackling this development a few years ago. We have recently decided to invest in a facility for the staked-needle syringe (Figure 7) – and the necessary equipment is due to be installed this year. The OXYCAPT™ syringe with a needle has some special features:
- tungsten-free
- glue/adhesive-free
- available in several gauges and needle lengths
- ultrasonic welding of syringe barrel, adapter and needle
- needle conforms to ISO 7864
- syringe conforms to ISO 7886-1.
BENEFITS FOR BIOLOGICS
We would also like to share the latest data from our drop testing of syringes. In a study based on ISO 11608-1:2014 (requirements and testing methods for needle-based injection systems), 20 gamma-sterilised OXYCAPT™ 1 mL long syringes and existing type 1 glass syringes were dropped from a height of one metre three times (horizontally once and vertically twice) on to a steel plate. Although 90% of glass syringes were broken, no breakage was observed among the OXYCAPT™ syringes (Table 2).
| Samples | Numbers of Breakage at 1st Testing (for whole parts) |
Numbers of Breakage at 2nd Testing (for flange part) |
Numbers of Breakage at 3rd Testing (For lure part) |
Numbers of Syringes without Breakage through 3 Testing |
| OXYCAPT™ | 0/20 | 0/20 | 0/20 | 20/20 |
| Glass | 12/20 | 10/20 (From 1st testing: 5/8) (New: 5/12) |
2/20 (From 1st testing: 1/3) (From 2nd testing: 1/7) (New: 0/10) |
2/20 |
Table 2: Drop testing of syringe.
CONCLUSION
In conclusion, OXYCAPT™ has been developed to solve some of the problems facing the pharmaceutical industry in creating syringes and vials suitable for the delivery of biologics, including via wearable devices. In addition to the special features of COP, such as a high water vapour barrier, high breakage resistance, very low extractables and low protein adsorption, OXYCAPT™ can also provide a high oxygen and UV barrier.
We believe OXYCAPT™ brings numerous considerable benefits to the rapidly growing field of biologics and regenerative medicines.

