To Issue 127
Citation: Meliniotis A, “Making the Connection Between Efficacy and Environmental Sustainability”. ONdrugDelivery, Issue 127 (Nov 2021), pp 33–36.
Andreas Meliniotis looks at the factors that should be considered when designing devices with a view to sustainable connectivity.
“By carefully selecting design concepts, materials and manufacturing processes, environmental impact can be minimised whilst improving adherence.”
For any developer of an inhaled drug delivery device, the key design focus is on the user and the intended use to ensure that patient needs are met. There are, however, other needs that should be considered, particularly those of wider stakeholders across the supply chain, including the manufacturer, prescriber and payer. Considering these wider needs may alter the attractiveness of different design concepts by modifying the importance of certain attributes.
Evaluating all these stakeholder needs can also result in conflicting or contradicting requirements. Understanding these, and taking steps to reduce their impact, allows innovators to choose design concepts that meet the demands of the market as a whole.
When designing products, giving consideration to user and environmental needs at a conceptual level can increase the effectiveness of the medication by attaining a high level of confidence in the measured data, while achieving a minimised level of environmental burden. This will ultimately lead to a more attractive product proposition prior to progression into full development – and, potentially, products with more longevity and lower risk throughout the development, commercialisation and market lifecycle.
The effectiveness of any treatment is a combination of many factors, most notably patient adherence to the dosage regimen and, in the case of complex products, the correct use of drug delivery devices by the patient. The lack of adherence and/or patient-use error can seriously compromise treatment outcomes.1 Ineffective treatment is widely understood to be a key contributor to increased levels of disease exacerbations and/or poor clinical outcomes, which clearly have a large environmental impact due to the burden on health systems dealing with hospitalisations. By carefully selecting design concepts, materials and manufacturing processes, environmental impact can be minimised whilst improving adherence, which potentially improves clinical outcomes2 and allows products to be cost effective at point of manufacture and supply.
CONNECTED DRUG DELIVERY
“Environmental requirements are likely to be in direct competition with those requirements related to user needs or the supply chain.”
As their sophistication increases, delivery devices are likely to be increasingly designed with connectivity in mind, which could open up possibilities for integrated connectivity or high-functionality add-on devices. Currently, connected drug delivery is achieved predominantly through the use of add-ons to existing approved devices with a low level of functionality – or as integrated connected systems with higher levels of functionality. Current examples of this are pressurised metered dose inhaler (pMDI) add-on devices, which sense the user pushing the pressurised canister to release the medication.
Although effective, the functionality of such devices is limited, as the base device has not been designed with connectivity in mind, so the options for communication between devices are restricted. Additional features such as orientation, shaking regimen or breathing duration sensing can now be easily included, often as standard with the integrated logic controller, and can offer useful insights that help to interrogate correct inhaler usage and give feedback to the patient.
Connected devices can have a positive impact on adherence and can identify user errors. However, these systems are costly, both financially and environmentally, and therefore should be applied only where needed most. For example, whereas insulin injections for diabetes generally have a high level of adherence because of immediate severe medical outcomes, an asthma maintenance therapy may exhibit poor adherence as there is no immediate, obvious decline in health. Using a connected device for the latter can provide a high level of monitoring, giving an early trigger for intervention to prevent uncontrolled disease and potential hospitalisation.
Some therapies can be affected significantly by user technique – and identifying this can have a dramatic effect on the efficacy of a treatment, particularly if it prevents a patient being prescribed a medication at a higher dose or additional medication to compensate for poor technique. Connected devices can provide both feedback and metrics to encourage patients to comply, while measuring physiological effects and reporting to the patient and the clinician can also help monitor the effectiveness of the medication, which can provide financial benefits in terms of reimbursement.
It is also important to consider the requirements of other parties or institutions that are involved throughout the supply chain. For example, attributes that may be highly important to the manufacturer include cost of goods, time to market and manufacturability. Whereas those relating to the clinician include effectiveness of treatment, effective disease management and market availability. These wider design requirements are unlikely to completely align with the user needs in all cases but clearly require consideration early in the design process.
Environmental requirements are likely to be in direct competition with those requirements related to user needs or the supply chain. For example, changing a propellant in a pMDI could require significant investment by the manufacturer; and including connectivity functionality could increase the cost of goods (both plastic and electronics) leading to higher levels of energy used during manufacture, and greater quantities of waste materials being discarded at the end of use.
GOOD DESIGN PRINCIPLES
By employing good design principles, it is possible to meet the requirements of both the end user and the wider stakeholders. For example, a device with a simple and easy-to-use interface and a low component count and volume can minimise both the cost of goods and the impact on the environment. The same can be achieved by using efficient manufacturing equipment, such as hydraulic rather than electric plastic injection moulding machines, and siting manufacturing facilities close to raw material suppliers to reduce transport costs.3
An analysis of the energy consumption related purely to plastic component manufacture shows how different ways of splitting the functionality in a device and the use of efficient injection moulding machines can alter the energy requirements for an annual therapy. Table 1 illustrates a monthly dry powder inhaler (DPI) combination product with either an integrated (option 1) or a clip-on connectivity unit (options 2 and 3), with different types of battery arrangements. These options lead to different lengths of a device’s lifespan – and hence different levels of energy required to manufacture the plastic components for an annual treatment. A re-usable handheld nebuliser-type device (option 4) is also considered, where it is sensible to replace the minimum level of plastic required for hygiene or performance considerations. The levels of energy are the average without considering raw material production.
| Option | Device type | Plastic (g) | Devices per annum |
Plastic per annum (kg) |
Hydraulic | Hybrid | All-Electric |
| 1 | Integrated connectivity disposable DPI device | 89 | 12 | 1.07 | 20.60 | 14.19 | 13.77 |
| 2 | Disposable, combination product device |
62 | 12 | 0.74 | |||
| Connectivity module with non-rechargeable battery |
27 | 4 | 0.11 | ||||
| Total | 0.85 | 16.44 | 11.56 | 10.98 | |||
| 3 | Base, disposable, combination product device | 62 | 12 | 0.74 | |||
| Connectivity module with changeable or rechargeable battery |
27 | 0.5 | 0.01 | ||||
| Total | 0.76 | 14.61 | 10.28 | 9.76 | |||
| 4 | Integrated connectivity, re-useable, medical device with changeable or rechargeable battery |
70 | 0.5 | 0.04 | |||
| Replacement mouthpiece and nebuliser head |
55 | 12 | 0.66 | ||||
| Total | 0.70 | 13.41 | 9.43 | 8.96 |
Table 1: Levels of energy consumption during the manufacture of plastic components for different types of connected devices.
By splitting the device functionality such that the re-usable functional elements are retained for as long as possible, and by using efficient manufacturing methods, the energy required can be minimised. Table 1 shows that a device manufactured by option 1 on a hydraulic press would use more than twice the amount of energy as option 3 that is manufactured on an all-electric press, for instance.
The ultimate goal is to describe an ideal product by taking all these requirements into consideration, then to address those conflicting and contradicting requirements at a conceptual level, thereby arriving at a theoretical direction that can inform decisions in the top-level product design stages.
Connectivity and sustainability have contradictory requirements. But techniques such as TRIZ (the theory of inventive problem solving) can be used to explore available options at the concept level and to define the desired conceptual direction (Figure 1). The TRIZ analysis highlights the need to include systems or materials only in the components or units for which they are needed – and recover parts of the system that can be re-used.
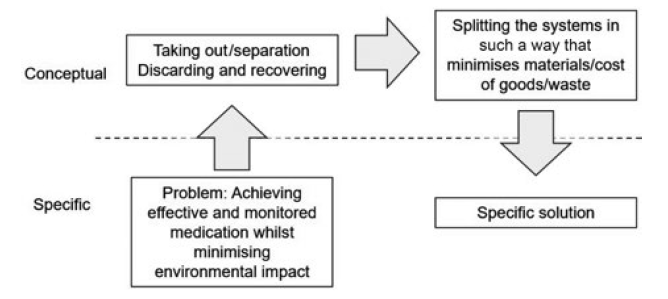
Figure 1: TRIZ methodology applied to connectivity and sustainability.
An “ideal” product would maintain a high level of functionality in a package that minimises the environmental impact, both through accurate analysis and recording of the correct use and by the minimised level of additional material and waste. To achieve this, the base DPI would need to be designed with the connectivity unit in mind to ensure the necessary level of sensing can occur – but packaged in such a way that the addition of a connectivity unit does not reduce the ease of use of the device.
Focusing on minimising the environmental impact at a conceptual design level and recognising that increasing functionality can increase levels of adherence and reduce use errors, which in turn will improve therapy and hence reduce levels of medical intervention, can potentially have a net positive impact on the environment.
“Vectura’s state-of-the-art, breath-actuated nebulisers with guided inhalation have been developed to improve lung delivery for inhaled small molecules and biologics.”
VECTURA: INTEGRATED PRODUCT DEVELOPMENT SERVICES
Vectura integrates formulation, device and development capabilities to offer a broad range of services to help customers bring inhaled medicines to market. The company has development expertise across a range of platforms, including capsule- and blister-based DPIs, pMDIs and smart nebulisers. This allows a “device agnostic” approach to be taken to meet the needs of the customer, the development programme and, ultimately, the patient.
In early development, a unit-dose capsule or blister device or a nebuliser may offer benefits in terms of speed and cost, whilst a multidose DPI, pMDI or nebuliser may be required as a commercial-ready platform to support a later-phase programme.
DPI Technology
Unit-dose capsule inhaler technology offers flexible dosing and a fast-to-clinic option in early development. Vectura can support capsule-based DPI programmes from preclinical development to small-scale commercialisation.
Vectura’s blister-based DPI platforms offer customers a range of options with simple design, intuitive user interfaces, low component count and access to devices used in products that are globally approved for multidose applications.
pMDI Technology
pMDIs remain an important device option for many patients. With extensive expertise in pMDI development and lifecycle management, including flutiform® and breath-actuated flutiform® K-haler®, Vectura can assist in the development of new pMDI products or optimise liquid formulation and device performance, based on either standard or novel propellants.
Nebuliser Technology
Vectura’s state-of-the-art, breath-actuated nebulisers with guided inhalation have been developed to improve lung delivery for inhaled small molecules and biologics, with the aim of achieving better clinical outcomes and/or shortened treatment times.
A patient’s breathing pattern can impact the efficiency of drug delivery to different regions of the lung. Control of the inspiratory flow rate, the inspiratory volume of the inhalation and the timing of aerosol delivery during the inspiration can materially affect how much drug accesses the central or peripheral parts of the lungs. This is the basis of Vectura’s proprietary, smart nebulisation technology.
Vectura has developed the FOX® nebuliser, which is a handheld, breath-actuated, smart mesh device, combining small droplet size, controlled flow rate and guided inhalation to offer high-performance drug delivery to the lungs. Small aerosol droplets are delivered consistently via silent, vibrating mesh technology. During inhalation, the FOX device guides the patient to inhale slowly and deeply, whilst the airflow resistance is varied to ensure a constant flow rate during inhalation, and an illuminating mouthpiece provides patients with a visual cue to ensure they inhale at the correct rate.
Vectura also offers the AKITA® JET device, which is a desktop, breath-actuated inhalation system that uses proprietary positive pressure technology to assist drug delivery to the lungs. The AKITA JET also guides the inhalation manoeuvre, providing real-time feedback and inhalation performance information to the patient or caregiver.
REFERENCES
- George M, Bender B, “New insights to improve treatment adherence in asthma and COPD”. Patient Prefer Adherence, 2019, Vol 13, pp 1325–1334.
- Jardim J, Nascimento O, “The Importance of Inhaler Adherence to Prevent COPD Exacerbations”. Med Sci (Basel), 2019, Vol 7(4), p 54.
- Thiriez A, “An Environmental Analysis of Injection Molding”. Massachusetts Institute of Technology, Thesis, May 2006.

