To Issue 127
Citation: Clayton J, Pinto J, Zellnitz S, “Powder Characterisation: a Critical Tool for the Evolution of Inhaled Drug Delivery”. ONdrugDelivery, Issue 127 (Nov 2021), pp 60–63.
Joana Pinto, Sarah Zellnitz and Jamie Clayton discuss the benefits of multi-faceted powder characterisation in dry powder inhalation formulations and how it can support efforts to achieve net zero emissions.
“Switching to DPIs is an attractive strategy for companies working towards net zero.”
Inhaled drug delivery technology has matured considerably in recent decades but there are now new issues to address. Efforts being made towards net zero emissions have highlighted the environmental impact of inhalers, notably metered dose inhalers (MDIs), while the covid-19 pandemic has triggered the evaluation of inhaled therapies for both prevention and treatment. Addressing these new challenges calls for further evolution of inhaled drug delivery, which will simultaneously help with longer-standing goals, such as with the development of efficacious therapies for rarer infectious lung diseases and the better exploitation of the pulmonary route for systemic drug delivery.
With respect to environmental impact, there is much to recommend dry powder inhalers (DPIs). The hydro fluoroalkanes (HFAs) used as propellants in MDIs have a global warming potential 1430–3200 times that of CO2,1 which results in commercial MDIs having an estimated carbon footprint per actuation around ten times that of DPIs (based on full product lifecycle analysis).2 Switching to DPIs is an attractive strategy for companies working towards net zero. However, relying on a complex device to achieve acceptable drug delivery makes device recycling difficult. As such, developing simpler devices in combination with formulations engineered for optimal aerosolisation is a more sustainable option.
Covid-19 proceeds by rapid viral replication following infection via the respiratory tract, making the targeted, inhaled delivery of antiviral drugs potentially beneficial. While remdesivir has been successfully delivered intravenously, in hospitals, trials are underway to explore delivery by DPI or nebuliser,4 with DPIs being the easier option for routine, community-based use. Comparable trials are also in place for hydroxychloroquine (HCQ), as consensus grows that the lung airway concentration reached is too low to be effective when administered under a safe oral dosing regime. In place of twice-daily oral doses of 400 mg, the intended DPI dose is just 20 mg.4 Targeted delivery is expected to result in an efficacious concentration with this much lower dose, but 20 mg is still relatively high in inhaled drug terms.5
The HCQ trials are being conducted using the Cyclops™ inhaler (Pure IMS, Roden, the Netherlands), a device with the efficiency to deliver high drug loads with minimal throat irritation. New devices, in combination with particle engineering, have extended the dosage range accessible with DPIs to >100 mg.5 This is an important trend, as increasing numbers of large molecules enter the respiratory drug pipeline, including proteins, oligonucleotides, antibodies and nanobodies, and for the treatment of infectious diseases, as exemplified by the use of high doses of tobramycin for the treatment of Pseudomonas aeruginosa infection in cystic fibrosis.5 Going forward, there is an expectation that formulators will need to deliver ever higher drug loads with powder formulations that are low density and exhibit poor flowability.3,6
In this article, we show how multi-faceted powder characterisation can support effective DPI formulation within this changing landscape. Referencing studies led by researchers from the Research Center Pharmaceutical Engineering GmbH (Graz, Austria),7,8 we illustrate how measuring dynamic, shear and bulk powder properties delivers understanding that is inaccessible with traditional techniques to aid formulation optimisation.
“Parameters that provide insight into aerosolisation behaviour, including particle-particle interactions, such as those between active and carrier, help formulators to build the understanding needed to
engineer superior performance.”
WHAT CAN BE MEASURED: QUANTIFYING FORMULATION PROPERTIES
The central challenge of DPI formulation is to deliver fine active particles, 1–5 μm in size, within the constraint of minimal energy input. The passive nature of most DPIs means that drug dispersion is driven solely by the inhalation manoeuvre of the patient, but inhalable actives tend to have a large specific surface area and correspondingly high cohesivity. Carriers are routinely used to create formulations with improved flowability and dispersibility, but this introduces the complications of attachment and detachment of the active, during manufacture and dose delivery respectively.
Parameters that provide insight into aerosolisation behaviour, including particle-particle interactions, such as those between active and carrier, help formulators to build the understanding needed to engineer superior performance. Particle properties – such as size, shape, specific surface area and surface morphology – are highly relevant, but so too are bulk powder properties, such as flowability. Unfortunately, traditional techniques for powder flow testing, such as tapped density methods, often exhibit poor repeatability and sensitivity, limiting their value for DPI applications. In contrast, dynamic powder testing with a powder rheometer delivers high sensitivity flowability measurement under a range of test conditions.
Figure 1 illustrates how values of basic flowability energy (BFE) and specific energy (SE) are generated by dynamic testing. BFE values are highly reproducible and can be used to securely differentiate formulations classified as identical by traditional techniques.9 Dynamic testing is also uniquely valuable with respect to quantifying a powder’s response to air – a critical characteristic for DPI formulations. Measuring flow energy as air flows up through the sample at a defined velocity, through to the point of fluidisation, generates values of aerated energy (AE), which have been correlated with fine particle dose (FPD)/fine particle fraction (FPF) – the dose or fraction likely to deposit in the lung due to its size, typically <5 μm.9,10
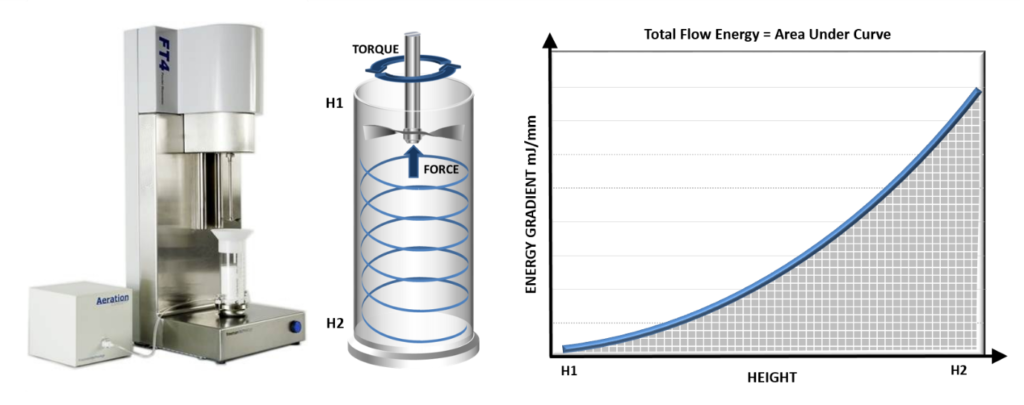
Figure 1: Measuring the torque and force acting on the blade of a powder rheometer as it traverses down or up through the sample generates values of BFE and SE, respectively.
Alongside dynamic testing, powder rheometers, as exemplified by the FT4 Powder Rheometer®, offer shear cell analysis and bulk property measurement – density, compressibility and permeability – substantially enhancing their value for DPI formulation characterisation. Permeability values are especially relevant since these also elucidate response to air, quantifying resistance to airflow and the ease with which a powder releases air, both of which can be pertinent to DPI behaviour.
In summary, using a sophisticated powder rheometer not only produces more sensitive and reliable data than traditional techniques, but also generates multiple properties, helping formulators to build a stronger knowledge base to support faster, more efficient DPI development, as the following studies demonstrate.
“Our understanding of formulation behaviour needs to improve to effectively shape DPI technology to deliver the performance required.”
BUILDING BETTER MODELS: HARNESSING THE POWER OF IN SILICO EXPERIMENTS
A cost-effective way to accelerate DPI development is to increase the use of in silico models. Maximising the relevance and utility of both in vitro techniques and in silico models reduces reliance on in vivo testing, which is slower, more complex and more costly. Research reported by Pinto et al (2021)7 demonstrates the potential value of a combined in vitro-in silico approach. In this study, four lactose carriers were characterised in terms of specific surface area and porosity (gas adsorption, Tristar II 3020 (Micromeritics)), density (helium pycnometry, AccuPyc II 1340 (Micromeritics)) and surface morphology/shape (scanning electron microscopy, Zeiss Ultra 55 (Zeiss, Oberkochen, Germany)).
Samples of each carrier were blended with 2% w/w salbutamol sulphate (SS) and all carriers and blends were characterised in terms of particle size (laser diffraction by pressure titration, HELOS/KR (Sympatec, Clausthal-Zellerfeld, Germany)), tensile strength and hardness (PTB 311E (PharmaTest, Hainburg, Germany)). Permeability, compressibility and shear properties were also measured (FT4 Powder Rheometer®, Freeman Technology). The aerodynamic performance of the blends was assessed as a function of airflow rate using both capsule and multi-dose/reservoir DPI devices.
A physiologically based pharmacokinetic model (PBPK) model of in vivo SS uptake was developed, predominantly from published data, although measured values of drug solubility and density were also used. Such models consider the clinical impact of patient physiology, an important factor in drug delivery by DPI, and are becoming an increasingly popular tool in drug development. Once validated, the model was used with the aerodynamic performance data for each of the four blends to predict in vivo performance. By identifying correlations between in vivo performance and carrier/blend properties via statistical analysis, it was then possible to determine the most relevant powder properties for carrier characterisation and selection.
Aerosolisation and drug delivery parameters, including the extent of drug deposition in the lung relative to the extra-thoracic region and FPF, were found to correlate with shear properties. In addition, for the capsule device only, correlations were observed between key pharmacokinetic properties such as maximum drug concentration level in the plasma and the bulk powder properties of permeability and compressibility. Possible rationalisations for these observed correlations are provided in reference nine.
This discussion affords only the briefest of insights into this detailed piece of work, but it highlights certain key points. Firstly, there is scope to use in silico models as learning tools in combination with relevant powder property measurement to support the selection and engineering of carriers to deliver enhanced in vivo performance. Secondly, access to multiple test capabilities was vital for this work since shear, permeability and compressibility data were all recommended for ranking carriers for different DPI devices. Shear testing alone would not have been able to perform the same role with respect to supporting carrier selection.
IMPROVING AEROSOLISATION: ENGINEERING HIGH-PERFORMANCE CARRIERS
A further study illustrates the use of advanced powder characterisation techniques in the development of novel carriers with superior drug delivery performance.8 Engineering carrier particles to which active particles will readily attach and then detach during aerosolisation calls for a precise control of carrier morphology that, in turn, relies on having information on which to base particle engineering decisions. In this study, three distinct types of spherically agglomerated lactose were prepared using the quasi-emulsion solvent diffusion method of spherical crystallisation. These carriers were characterised in terms of particle size, surface morphology, specific surface area and porosity (techniques and instrumentation as described for the preceding study). Bulk and tapped density were determined (PT-TD200 (Pharmatest)) to produce Hausner ratio values, and dynamic properties (including BFE and AE), shear properties, permeability and compressibility were all measured (FT4 Powder Rheometer, Freeman Technology). Characterisation was also carried out with a commercially available lactose grade Lactohale 100 (DFE Pharma) for comparative purposes.
Blends of each carrier with 2% w/w and 5% w/w SS were then produced, and aerosolisation performance was assessed via in vitro testing (cascade impaction) using a capsule-based DPI device. A principal components analysis was carried out to determine which carrier properties correlated most strongly with aerosolisation performance. The results showed that, with this system, carriers with higher specific surface area, higher fines, lower permeability and higher cohesion resulted in higher FPFs. Higher BFE values were associated with lower FPFs. It is worth noting that Hausner ratio values were not observed to correlate with aerosolisation performance. In contrast, permeability was singled out for the strength of correlation with FPF and for its ability to effectively account for the combined impact of specific surface area, particle size and shape, surface roughness and morphology.
LOOKING AHEAD
DPI technology is attractive from an environmental perspective and has the potential for the delivery of high dosages – an increasingly important clinical requirement. However, the particle engineering involved in DPI formulation is complex and challenging. Our understanding of formulation behaviour needs to improve to effectively shape DPI technology to deliver the performance required.
Reliable, multi-faceted powder characterisation has an important role to play in extending the knowledge base for DPI formulation. Multiple studies suggest that dynamic, shear and bulk powder property measurements can help to elucidate DPI formulation behaviour, notably in response to air and aerosolisation behaviour. The recent studies reported here demonstrate the value of powder testing when it comes to optimising the use of in silico models – a vital tool for cost-efficient progress – and for the development of new carriers. Modern, advanced powder-testing techniques offer the sensitivity, flexibility and relevance required to deliver unique insight into DPI formulation behaviour and can directly support the development of this vital technology.
REFERENCES
- Pierce JMT, “Rapid response re: a more sustainable NHS.” BMJ, 2019, Vol 366: I4930.
- Panigone S et al, “Environmental impact of inhalers for respiratory diseases: decreasing the carbon footprint while preserving patient-tailored treatment.” BMJ Open Res Rev, 2020, Vol 7: e000571.
- Fernandez JV, Villax P, “‘Dry Powder Inhalers: Towards Effective, Affordable, Sustainable Respiratory Healthcare.” ONdrugDelivery, Nov 2019, Issue 102, pp 5–8.
- Humphries B et al, “Inhalation therapies for COVID-19.” Faculty of Pharmaceutical Medicine Blog, Jan 28, 2021.
- Hickey A, “Emerging trends in inhaled drug delivery.” Adv Drug Del Rev, 2020, Vol 157, pp 63–70.
- Petersson G, “Pipeline Trends and Challenges in Pulmonary Delivery.” ONdrugDelivery, Nov 2018, Issue 92, pp4–8.
- Pinto JT et al, “‘Understanding carrier performance in low dose dry powder inhalation: An in vitro-in silico approach.” Pharmaceutics 2021, Vol 13(2), p 297.
- Zellnitz S, Lamešic, D, Stranzinger S, “Spherical agglomerates of lactose as potential carriers for inhalation.” Eur J Pharm Biopharm, 2021, Vol 159, pp 11–20.
- Clayton J, “Reviewing current practice in powder testing.” Org Process Res Dev, 2015, Vol 19(1), pp102–109.
- Zhao Z, “Effect of powder properties on the aerosolization performance of modified mannitol carrier based on dry powder inhalation.” Poster presentation at AAPS 2017.

