To Issue 140
Citation: Frankza M, Khoury N, “The Innovations that are Helping to Tackle Challenges and Maximise Opportunities for Inhalation Solutions”. ONdrugDelivery, Issue 140 (Nov 2022), pp 34–36.
Marco Franza and Nadine Khoury look at how the latest digital technologies, manufacturing techniques and advances in packaging materials are combining to take the manufacture of inhalers to a new level in terms of the patient experience, financial improvements and sustainability gains.
The fast-track introductions of the various vaccines during the covid-19 pandemic has highlighted how the pace of drug development has increased significantly in recent years. Today, pharmaceutical and healthcare companies can bring many types of treatment to market more quickly.
At the same time, the drugs now being developed are becoming ever more sophisticated, in terms of their overall effectiveness and how they can be closely targeted to different patient groups. This enables the provision of more individualised care for patients, tailored to their specific needs and medical history. According to a Euromonitor report on the future of personalised healthcare, this patient-tailored approach is expected to reduce trial-and-error treatments and improve outcomes and healthcare cost effectiveness.1
“A new generation of models, which incorporate digital technology,
is able to improve adherence and monitor treatments more effectively to greatly enhance patient outcomes.”
The report also identifies how personalised healthcare systems are being driven by strong biotech and R&D sectors that are helping to speed up the development of these new solutions, combined with the experience of the pharmaceutical industry, which brings them quickly to manufacture and commercialisation.
Alongside this, the introduction of these precision medicines has been supported by the move to eHealth, telemedicine and remote diagnostics – a trend that was accelerated by the pandemic. Through the use of information management and information and communication technology, diagnoses can be carried out remotely, and care and treatment integrated into patients’ daily lives. Carers can also share relevant information more easily, and data can be analysed as part of disease management or a clinical trial.
In this way, patient engagement can be enhanced and improved, encouraging positive behaviour and ensuring greater adherence and compliance to treatments (Figure 1).Such sophistication presents additional challenges in terms of how these treatments are packaged, dispensed and administered. Each format needs to meet the precise requirements of the particular drug while also delivering the appropriate patient experience so that the drug can be taken easily and intuitively to maximise its effectiveness.

Figure 1: A new generation of inhalers with digital technology is seeking to improve inhaler adherence and technique.
In its ongoing development of solutions to deliver drugs effectively to the lungs, the medical device sector is no stranger to innovation. The first pressurised metered dose inhaler was introduced in 1956 and, since then, we have seen continual enhancements and improvements to the technology, as well as the introduction of dry powder inhalers.

Figure 2: Advanced manufacturing techniques are critical to the capabilities of the latest inhalers.
Much of the success of today’s inhalers is a result of the advanced manufacturing techniques that have been introduced over the years (Figure 2). High-precision moulding and assembly are required for the complex and technical parts, some of which are microdimensional. Internal coatings must deliver product protection and stability.
It is therefore quite sobering to learn that, despite these many advances since 1956, the number of inhaler errors caused by inadequate adherence and technique has hardly changed at all during this time. Specifically, up to 94% of patients still make critical mistakes using their inhalers.2 Another study found that adherence to asthma and chronic obstructive pulmonary disease treatments was as low as 32%, meaning two out of three people were taking their medicine incorrectly.3
It is this problem that the latest technical inhaler innovation is seeking to overcome. A new generation of models, which incorporate digital technology, is able to improve adherence and monitor treatments more effectively to greatly enhance patient outcomes.
The new inhalers incorporate built-in sensors with digital capabilities that allow them to track inhaler use. This information is shared with a companion app, which then provides personalised guidance to each patient. The use of the app reminds patients when to take a dose and provides tips to help them self-medicate more effectively. Patients can also share their data with healthcare providers, either remotely or in person, to enable data-driven treatment adjustments.
A typical app is able to track when and how a patient inhales a dose and provide an alert if a dose is missed. It can monitor inhaler orientation during inhalation and capture estimates of inhalation parameters. Through this, personalised learning content can be prepared with health forecasts and insights. Patients can build better inhalation techniques, learn to identify and avoid what triggers their symptoms and track their breathing to identify signs of improvement or worsening of their condition.
For one app already on the market, Amiko‘s Respiro™, a seven-month randomised control trial generated a 43% improvement in medication adherence, a 61% reduction in inhaler technique errors and a +5.9 improvement in asthma control (Figure 3).
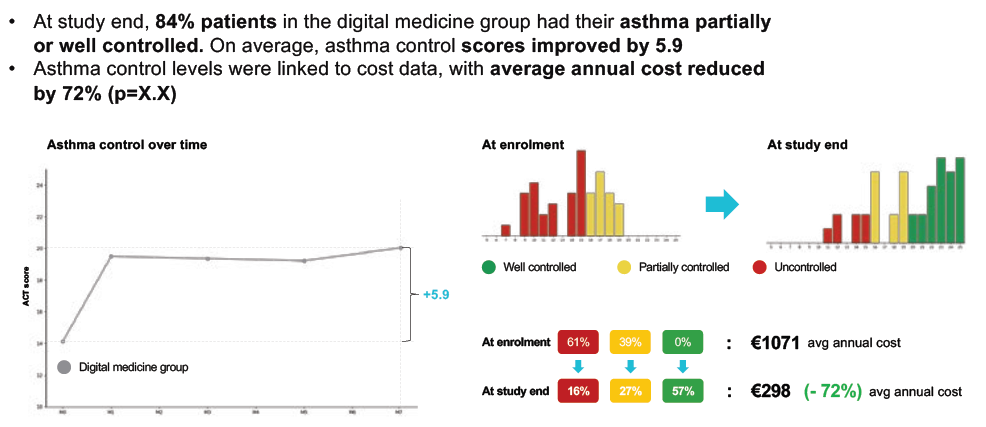
Figure 3: Positive improvement in asthma control scores in a trial of the Amiko Respiro™ app.
“Alongside these continual improvements in design and technology, the drive for more sustainable solutions is also becoming a critical factor in inhaler design.”
Importantly, these digital features can be introduced without compromising on the technical design and intricate manufacture of the inhalers. This ensures that they retain both their ability to dispense the medicine accurately and their user-friendly features, particularly in terms of suitability for all age groups.
While these benefits are primarily focused on improving patients’ quality of life, they have equally important financial implications. A recent study by York Health Economics Consortium and The University of London’s School of Pharmacy estimated that the resulting net benefit associated with greater compliance for asthma treatment was £2,250 per patient, leading to savings of £90–130 million per year.4
Similarly, Health-monitoring.com estimated the costs associated with imprecise dosing of medicines to be around US$765 billion (£680 billion), which represents some 30% of total spend on healthcare.5 Alongside these continual improvements in design and technology, the drive for more sustainable solutions is also becoming a critical factor in inhaler design.
This is not to say that this is something completely new to medical devices. The light weighting of products to reduce the amount of material required to produce them has long been a key factor in new inhaler designs. Here, the critical consideration has been to lower the weight of the dispenser while ensuring that it remains fully functional and user friendly, and continues to deliver the best patient experience.
Another important consideration for any product design has been to improve its recyclability. This has led to the development of more mono-material solutions, made in materials such as polyethylene (PE) and polypropylene (PP), both of which are now widely recyclable.
Alongside this has been the introduction for pharmaceutical and healthcare applications of ISCC Plus certified packaging and plastic components that contribute to a circular economy approach.
ISCC is a globally applicable sustainability certification system, covering all sustainable feedstocks, including agricultural and forestry biomass, circular materials and renewables, based on advanced recycling mass balance. Mass balance is an accepted and certified protocol that documents and tracks recycled content from supplier through to final delivery to customers.
The availability of this advanced recycled resin in both circular PP and PE, which is free from harmful contaminants and therefore suitable for food and pharmaceutical applications, provides the opportunity for the development of solutions for drug delivery applications that contain a high percentage of recycled material, from 30% to 100%. Crucially, these can be for products where this has been difficult to achieve previously.
Increased consumer spending on healthcare, demand for customised services and an ageing population are all driving demand for personalised healthcare services. For inhalation treatments, the transition to precision medicine and to outcome-based care provides great opportunities to improve patient technique and adherence through the adoption of digital technologies, and the health and financial benefits that this can bring are plain to see. And by focusing on both human- and environmental-centred design, the medical device industry can devise solutions that benefit both people and the planet.
REFERENCES
- “Future of Personalised Healthcare”. Research, Euromonitor International, Apr 2022.
- Lavorini et al, “Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD”. Respir Med, 2008, Vol 102(4), pp 593–604.
- Rolnick SJ et al, “Patient characteristics associated with medication adherence”. Clin Med Res, 2013, Vol 11(2), pp 54–65.
- “Visualizing the Cost of Health Care”. Research report, Health Monitoring, Oct 2014.
- Trueman P et al, “Evaluation of the scale, causes and costs of waste medicines. Report of DH funded national project”. York Health Economics Consortium and The School of Pharmacy, University of London: York and London, 2010.

