To Issue 140
Citation: Beller K-D, “CLIPhaler – Inhalation Device Design Reduced to the Maximum”. ONdrugDelivery, Issue 140 (Nov 2022), pp 60–63.
Klaus-Dieter Beller discusses the advantages of the inhalation route of administration, how his principle of design simplicity has informed the design of novel inhalation devices and introduces CLIPhaler, his latest and simplest device.
POTENTIAL OF INHALATION AND NASAL ADMINISTRATION
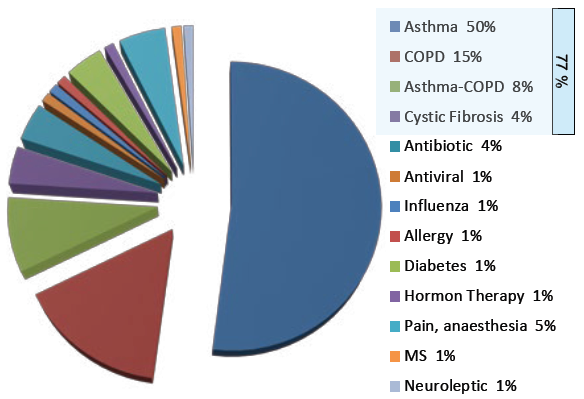
Figure 1: Breakdown of inhalation market share by indication.
While a significant majority of therapeutic agents remain administered either orally or via injection, the inhalation route remains a key delivery pathway for a number of therapeutics, especially when treating diseases of the lung. Worldwide, the most important indications for inhaled drugs are asthma and chronic obstructive pulmonary disease (COPD). Over 70% of the inhalers on the market are used for these two conditions (Figure 1). On the other hand, nasal drug delivery – both in powder and liquid form – accounts for less than 5%.
The pharmacological potential of the inhalation and nasal routes of administration is far from being fully realised. The well-vascularised mucosal membranes of the pharyngeal region and the nasal area are easily accessible, and yet relatively few delivery methods and therapeutics have been developed to target them. Furthermore, the inhalation route only becomes more relevant as an increasing number of patients develop a fear of injections and the oral route becomes increasingly difficult for newer, more sensitive drugs due to maldigestion, malabsorption and swallowing difficulties. This unmet need should be closed, or at least addressed, by new drug product developments.
Some novel devices have been developed in the sector, including the mucosal atomisation device by Teleflex (NC, US) and the Adapplicator “spraying ampoule” by Köhler Pharma (Alsbach-Hähnlein, Germany) – one of the author’s inventions. There have also been promising results from nanopatch microprojections, with recent studies showing a 10-fold increase inimmunisation by vaccines when applied through micro-array patches. From an immunological perspective, oropharyngeal application is particularly interesting, given its access to immunologically important transdermal cells. The Waldeyer’s pharyngeal ring or lymphatic pharyngeal ring plays a dominant role here.
“Inhalation and nasal administration offer a number of advantages, including relative ease of use and improved patient acceptance compared with injectable administration.”
Inhalation and nasal administration offer a number of advantages, including relative ease of use and improved patient acceptance compared with injectable administration. For sustained injectable use, pharmacovigilance must always be considered; injectable applications are associated with higher risks, such as needlestick injuries and misinjection, and side effects. In contrast, inhalation and nasal applications are noninvasive and not subject to first-pass metabolism. Furthermore, it has been demonstrated that nasal application is capable of directly reaching the central nervous system and bypassing the blood-brain barrier. Additionally, in many cases, mucosal applications have shown a faster onset of action and are suitable for both local and systemic treatments.
WHY IS THIS POTENTIAL NOT USED?
Inhaler development and production is complex, expensive and time-consuming. This is true for both passive and, to an even greater extent, active systems. Inhalers are usually quite complex in design, consist of several parts and frequently have complicated manufacturing processes. The expense and risk inherent in developing an inhalable therapeutic can only be justified if significant sales are expected, whether due to a high individual unit price or it being necessary for long-term maintenance.
The author has set himself the goal of meeting this unmet need by developing a simple, inexpensive and user-friendly dry powder inhaler (DPI). The stated objective was to keep the design as minimalistic as possible to expand its potential therapeutic areas and applications and make inhalation and nasal administration affordable for emerging economies. The motto for this project was “Reduce to the maximum”. In contrast to common DPIs, these minimalistic inhalers are designed to consist of only a few components to minimise tool, material and assembly costs. Over many years, the author has developed a number of inhalers according to this principle, such as the VALVEhaler (developed with Braunform, Endingen, Germany), PERLAMED – BLISTair (Perlen Packaging, Perlen, Switzerland), Papillon (Hovione) and CLIPhaler.
VALVEhaler
The VALVEhaler is a two-part DPI in which the powder carrier – the DRUGpod – is contained within a rotatable and exchangeable cylinder (Figure 2). The system is activated by rotation or pressure. The DRUGpod defines the requirements, such as deagglomeration, airway resistance and turbulence via its “interior life”. The dosing quantity can be adjusted via the height and diameter of the DRUGpod. The VALVEhaler can be designed for single use or with exchangeable cartridges for multiple use. Tandem or triple function can be realised by simple design modifications to the DRUGpod.
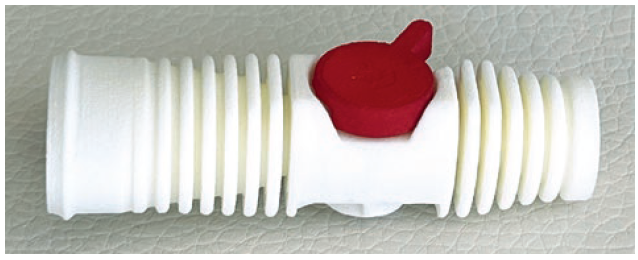
Figure 2: The VALVEhaler and DRUGpod.
PERLAMED – BLISTair
The world’s first cost-effective disposable inhaler, the PERLAMED – BLISTair is manufactured using a thermoforming process rather than the usual injection-moulding process. The BLISTair represents a new class of DPI:
- It can be manufactured and filled in a single manufacturing process without assembly
- The tacked manufacturing process is based on standard blister manufacturing and filling procedures
- The upper part (inlet and mouthpiece) can be formed from low-cost standard films, although laminates with a high barrier to water and/or oxygen must be used for the lower part with the powder chamber.
BLISTair can also be easily adapted to different requirements; by changing the relatively simple format parts, BLISTair can be adapted to different powder formulations and doses. Necessary changes to the functional geometry, such as resistance toairflow or bypasses, can also be introduced into the format parts. Operation of the device is extremely simple – with a simple pull on the peeling foil tab, the dosing chamber is opened and the BLISTair is ready for inhalation (Figure 3).
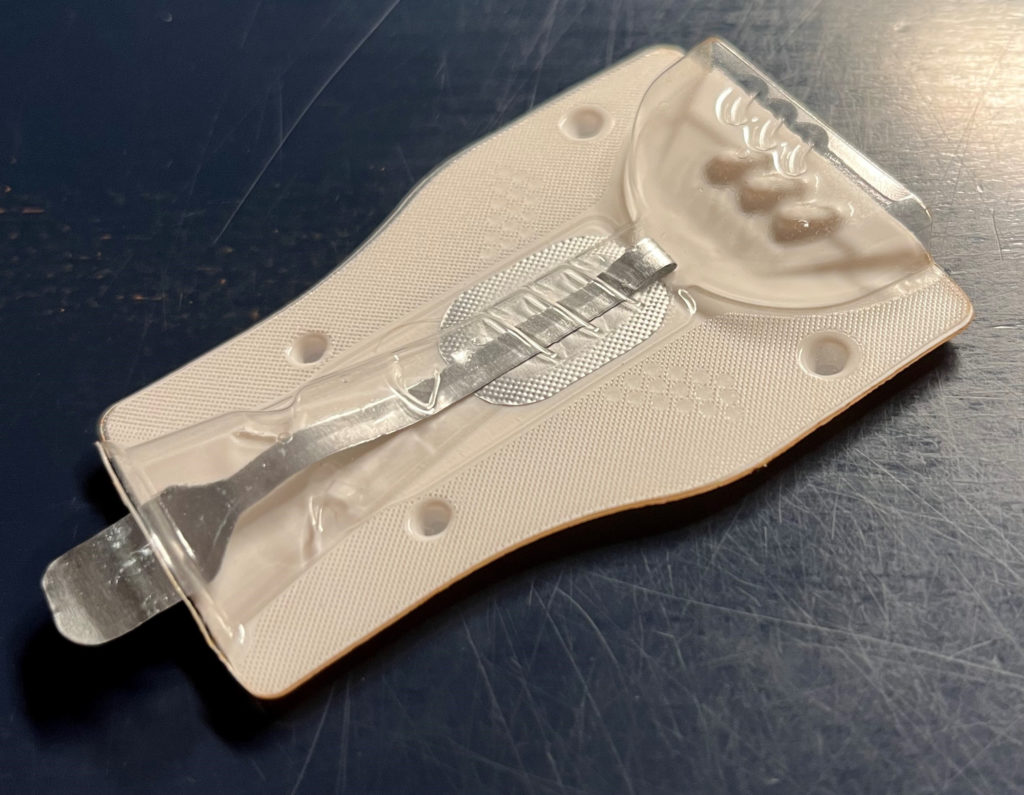
Figure 3: The PERLAMED – BLISTair.

Figure 4: The Papillon inhaler.
PAPILLON
The innovative single-dose, multi-use blister-based DPI Papillon represents a paradigm shift in inhaler development. Because it consists of a single, reusable plastic part, Papillon can achieve competitive single-dose costs at a fraction of the development cost and without the development risks inherent to complex, multi-part inhalers. The Papillon inhaler is extremely user-friendly; the patient inserts the blister, closes the device and inhales the dose. During this process, the prefilled blisters are automatically pierced. Due to its one-piece design, the Papillon can be adapted to a wide range of inhaled drug doses (Figure 4).
“Due to its one-piece design, CLIPhaler can handle a wide range of inhalable drug dosages in different formulations.”
CLIPhaler
The minimalist DPI CLIPhaler is a novel, patent-protected invention that contains no moving parts to assemble and consists of only one injection moulded part (Figure 5). CLIPhaler is extremely user-friendly; the user inserts the blister, unclamps the inhaler and the device automatically pierces the prefilled blisters. The inhaler is then ready for use. The transparent blister is always visible to the user, so the patient can easily check if they have taken the entire dose of their medication. If this is not the case, the patient can inhale again to ensure that they take their entire dose. This increases phamacovigilance and patient safety.
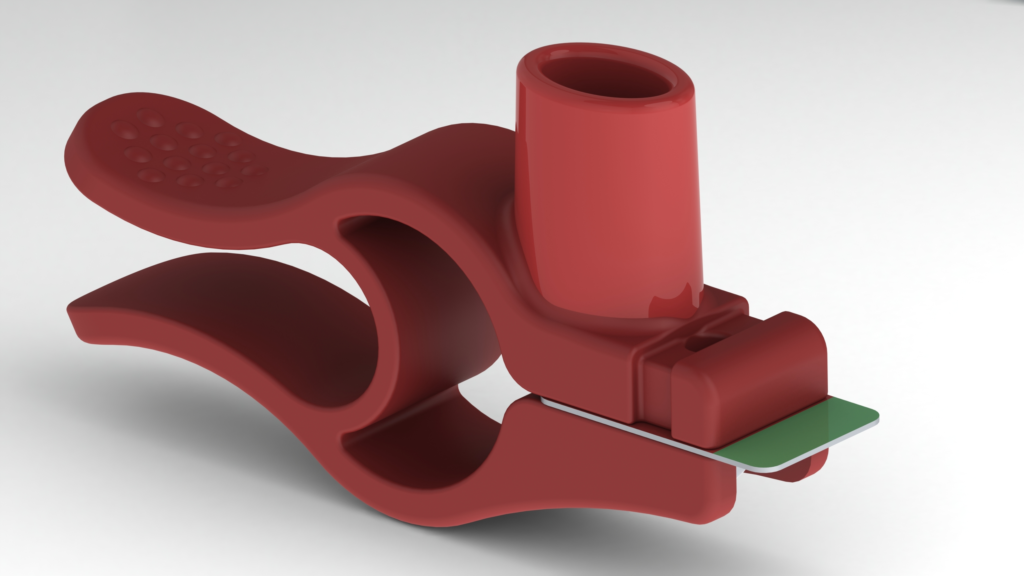
Figure 5: The CLIPhaler, a novel one-piece inhaler design.
Due to its one-piece design, CLIPhaler can handle a wide range of inhalable drug dosages in different formulations. Necessary changes to the functional geometry (breathing resistance, flow changes, bypasses) can be introduced easily, quickly and cost-effectively with only two mould inserts. This means that the interaction between powder and device can be quickly tested in the early development phase.
One of the mould inserts determines the orientation of the blister, while the fork shape defines the circumference and outer contour of the blister. The blister can have round, oval or asymmetrical shapes and be equipped with guides for positioning or fixing. The reusable-disposable function can also be defined via the fork-shape insert.
In addition to the piercing function, the second mould insert characterises the aerodynamic function of the spikes. The specially developed piercing mandrels not only enable debris-free piercing of the aluminium foil, but also create a unique swirling effect in the blister due to special design features. The blister therefore forms a functional part of the deagglomeration and aerosolisation of the inhaled powder.
The multi-edged hollow mandrels modulate the flow energy and flow directions depending on the grind, orientation and angular degrees. This is reminiscent of turbines and drive blades. The fluid flow is deprived of part of its internal energy by the laminar flow around the spikes, which is then transferred to the powder present in the blister. This improves deagglomeration and turbulence. Depending on the size of the CLIPhaler, up to two mandrels can be integrated in the inlet area. Additionally, depending on if the running directions of the guide vanes are oriented in the same or opposite directions, fundamental differences can be induced in the flow pattern. The use of moulded inserts allows the mandrels to be designed as conical worm threads (cyclone, helix). Finally, the airway resistance can be regulated by the design of the spikes.
The design and volume of the blister is integrated into the aerodynamic function by imprinting an internal structure on the blister. The deagglomeration process can be further modulated by this structuring of the blister.
A series of internal tests with reference substances have shown that 100% emitted mass and an effective drug separation mechanism are achievable. The following test substances were transferred by hand into CLIPhaler blisters and sealed, then tested with third-generation CLIPhaler prototypes:
- Seebri Breezehaler (Novartis): capsule containing 44 μg glycopyrronium
- Rolenium (Elpen Pharmaceutical, Attica, Greece): blister containing 50 μg salmeterol and 250 μg fluticasone propionate
- Spiriva (Boehringer Ingelheim): capsule containing 18 mg tiotropium
- Diskus Viani (GSK): blister containing 50 μg salmeterol and 250 μg fluticasone propionate
- AirFluSas Forspiro (Sandoz): blisters containing 50 μg salmeterol and 500 μg fluticasone propionate.
For non-respiratory indications, the strictly defined fine particle fraction (FPF) of 3–5 μm does not play a decisive role; instead, uptake via the well-vascularised mucosa in the pharynx is sufficient. To reach the transdermal cells in Waldeyer’s pharyngeal ring or the lymphatic pharyngeal ring, an FPF of 1–10 μg should be sufficient. Current respiratory inhalers are designed for respiratory patients with impaired respiration; however, inhaled drug delivery for non-respiratory patients can assume that patients will have good-to-very-good lung function. These patients will be able to generate higher inhalation energy and can therefore better deagglomerate the drug.
Like most DPIs on the market, the CLIPhaler is “passive” – it has no additional energy source. However, an “active” function can be adapted for the CLIPhaler. Such an active function was already considered for the metered dose inhaler “Penhaler” (developed by the author and Braunform) in a similar way.
The unique technical possibilities and commercial advantages for CLIPhaler include:
- Simple, easy design and development process
- Low-cost prototyping and production
- No need for assembly, reducing the effort required for quality assurance and management
- Single component means low production costs – CLIPhaler is much cheaper than currently marketed asthma inhalers, which can consist of up to 23 components.
Furthermore, CLIPhaler is strongly patent-protected, and so represents an excellent opportunity for market differentiation. The author’s patent portfolio covering CLIPhaler is currently available for sale and the author is seeking interest in bringing CLIPhaler to market.
The development of simple and costeffective DPIs, capable of delivering a wide range of formulations and dosage volumes are indicative of the interest in using the inhalation route in new therapeutic areas. In addition to the classic respiratory indications, minimalistic inhalers, such as CLIPhaler, represent a real alternative to oral and parenteral drug delivery. Innovative new APIs and formulations are leading to an increasing interest in using inhalers for new indications. For example, currently, inhaled nanocarriers are being developed for targeted lung cancer therapy. Since nanocarriers can easily overcome mucosal barriers, their use as an inhaled medication also has great potential for treating other diseases, especially in the immunological field.
CONCLUSION
In summary, CLIPhaler presents an interesting option for both new and old drugs. Current considerations around drug repurposing are looking to develop more therapeutic options for both common and rare diseases, often by changing their delivery route. Given the potentially lower development costs and development times, drug repurposing has seen increasing levels of interest in recent years. CLIPhaler is well positioned to help realise the full potential of this movement. Perhaps in the foreseeable future, injections and tablets can be replaced with cheaper inhalable alternatives, and necessary and lifesaving medicines can be made available in economically weak regions and areas with less-developed infrastructure.
The CLIPhaler has been designed for easy, intuitive use with maximum effectiveness and a minimum of components using only standard production processes and materials. The simplicity of CLIPhaler offers the possibility of use as an innovative disposable or reusable inhaler. CLIPhaler can also be easily adapted for new indications and therapeutic areas that require much higher delivery doses than respiratory indications.
CLIPhaler has been designed for nasal use, with compliance and human factors at the forefront of its design. Simultaneous inhalation of different active ingredients (tandem inhalation) is easy to realise. Additionally, CLIPhaler’s low material usage per inhaler and its monomaterial design supports environmental sustainability. CLIPhaler represents the full realisation of the ideal “Reduce to the maximum”.
The CLIPhaler patent portfolio is currently available for sale, please contact the author for further information. E: [email protected].

