To Issue 140
Citation: Baschung Y, “Expanding the Design of Characterisation Studies for Accurate Handling-Error Assessment”. ONdrugDelivery, Issue 140 (Nov 2022), pp 88–91.
Yannick Baschung discusses the importance of a comprehensive and accurate assessment of patient handling errors when it comes to inhaled product development.
“For the prescriber, easy access to comprehensive information about device-specific critical handling errors is often missing.”
Up to 87% of inhaler users are not using their inhaler properly.1 Generally, simple, easy-to-use, breath-actuated dry powder inhalers (DPIs) are handled better by patients than metered dose inhalers (MDIs), which require shaking and breath-actuation co-ordination. However, the diversity of DPIs with respect to design, operating principles, order of handling steps, exact adherence to the order of handling steps and feedback to the patient means that there is significant complexity and the potential to confuse patients.
Effectively and representatively establishing the link between in vivo action and in vitro performance for orally inhaled products can represent a major challenge, as lack of treatment adherence, incorrect breathing techniques and misuse of inhalers by patients can critically affect the delivered dose and the lung deposition – and therefore the success of the treatment.
Several studies exist in which the prevalence of human error for each type of inhaler and category is described. However, the actual impact of some of these handling errors on the effective dose delivered to the patient by the inhaler remains unclear, as only common respiratory and handling errors are evaluated in vitro . For the prescriber, easy access to comprehensive information about device specific critical handling errors is often missing – complicating effective inhaler patient training.
HUMAN CAPABILITIES AND LIMITATIONS
When evaluating an inhaler device, the design of the product’s user interface should be assessed in human factors (HF) studies, which evaluate the ability of the patient to perform critical tasks and to understand the information presented to them by the packaging and labelling, such as product labels or instructions for use, and how that informs the patient’s actions – factors that are critical to the safe and effective use of the device. Consistent with a risk-based design and development paradigm, HF studies should identify critical tasks that, if performed incorrectly or not performed at all, would or could cause harm to the patient or user, where harm is defined to include compromised medical care.2
Validated HF studies should demonstrate that the final finished device’s user instructions maximise the likelihood that the product will be safely and effectively used by patients, for the intended uses in the intended use environments. Moreover, in situations where understanding the information provided by the device labelling is critical for using a product safely and effectively (for example, the user’s understanding of the diagrams), a study to assess the user’s understanding of such information – a knowledge task study – is appropriate.
An appropriate HF development programme will maximise the likelihood that the device’s user interface is safe and effective for use by patients in the intended use environments. However, HF studies are frequently limited to assessing the device only in the context of the intended use and use environment and are not sufficient to establish the reliability of the device instructions in real-world use. Therefore, additional in vitro product characterisation studies (PCSs) are necessary to support the robustness and performance of the device and its labelling.
THE LIMIT OF CHARACTERISATION STUDIES AND INSTRUCTION LEAFLETS
PCSs are required by regulatory agencies for all MDIs and DPIs to characterise the optimum performance properties of a drug product and to support appropriate labelling statements, thereby contributing to patient compliance. For DPIs, alongside various stability, storage and environmental simulation studies, regulators require data on device performance for specific handling and breathing situations, such as flow-rate variation and device orientation.
Determining the emitted dose (ED) and aerodynamic particle-size distribution of the ED as a function of different flow rates at constant volume can help to evaluate the device’s sensitivity to the differences in breathing profiles between patients of different age, gender and severity of disease. However, the outcome of such studies is often limited by the narrow range of flow rates tested and by the restriction to a constant volume.
Device orientation studies aim to demonstrate the performance of a DPI across various dosing orientations. However, these tests are generally limited to the likeliest scenarios of device orientation variations – +45° and -45° – and omit scenarios where severely ill and bedridden patients use their devices in vertical positions.
Handling errors common to most of the bestselling devices – such as failure to properly close the DPI before actuation, failure to release the piercing button while actuating, double piercing the capsule before actuation or shaking the loaded device – are often addressed in the device’s instruction leaflets. However, their impact on product performance is rarely investigated as part of PCSs. Selestini et al3 reported in their study on prescription bias and factors associated with improper use of inhalers that only 66% of DPI users received at least some instruction from their healthcare provider, regardless of whether the prescribing physician was a generalist or a pulmonologist. However, DPI users had more often read the instruction leaflet accompanying their inhaler compared with MDI users (72% and 55%, respectively), possibly to compensate for the lack of instruction by their physician.
However, the benefit of providing information, including written instructions, without any form of “hands-on” demonstration has been shown to be similar to that of not providing any information at all. Significant improvements in DPI handling technique were only observed when the educational intervention included a practical demonstration, which requires the prescriber to have a comprehensive knowledge of the device. On the other hand, poor inhaler technique, due to either a lack of instruction or leaflet reading, has been associated with more frequent hospital emergency visits, presumably reflecting a poorer control of the underlying respiratory disease.
ASSESSING THE POTENTIAL IMPACT OF HANDLING ERRORS
Van der Palen et al4 evaluated patient compliance to a purpose-designed checklist specific to HandiHaler® (Boehringer-Ingelheim) after patients had received only written information. As can be seen in Table 1, breathing-related handling instructions scored the lowest, followed by failure to orient the device properly while piercing the capsule.
| Step | HandiHaler Checklist Item | Item Score |
| 1 | Open top cover | 100.00 |
| 2 | Open mouthpiece | 97.7 |
| 3 | Peel open strip with capsule | 76.6 |
| 4 | Put capsule in inhaler | 93.3 |
| 5 | Close mouthpiece until click is heard | 88.3 |
| 6 | Perforate capsule with mouthpiece facing upward | 68.3 |
| 7 | Release the perforation button | 75.0 |
| 8 | Exhale to residual volume, not in mouthpiece | 75.0 |
| 9 | Mouthpiece between teeth and lips | 95.0 |
| 10 | Inhale slowly and deeply to make capsule vibrate | 23.3 |
| 11 | Hold breath for several seconds | 28.3 |
| 12 | Remove empty capsule | 83.3 |
| 13 | Close inhaler | 81.7 |
Table 1: Patient score (percentage of patients performing the checklist item correctly) of relevant HandiHaler checklist items.
“Poor inhaler technique, due to either a lack of instruction or leaflet reading, has been associated with more frequent hospital emergency visits.”
While these errors are usually considered by the PCS, the impact of other handling errors that could potentially impact the performance of the device, such as improper closing of the mouthpiece or not releasing piercing button, remains generally unknown. Moreover, the score for typical DPI handling errors, such as shaking the device with the capsule loaded or double piercing the capsule, were not evaluated in this study and their impact on thein vitro performance, which are presumably device specific, are also often unknown.
To investigate these unknowns, a study was undertaken to reproduce the respiratory and handling errors listed in Table 2 in vitro . The standardised HandiHaler in vitro testing flow rate of 39 L/min and volume of 2 L were used as a reference. Six replicate determinations were performed per ED determination and collected in a dosage unit sampling apparatus (DUSA). All determinations were evaluated by reversed-phase high performance liquid chromatography (HPLC). ED results were reported in percentages against the target delivered dose (TDD) of 10.4 μg reported in the Spiriva HandiHaler prescribing information.
| Test Parameter n° | Reproduced Errors | In Vitro Parameters |
| 1 | Forcefully and deeply inhale through the device | Inhalation flows : 10 – 20 – 100 L/min Inhalation volume: 2 and 4 L |
| 2 | Not closing the device correctly | Device remains slightly open (no “click” sound) during actuation |
| 3 | Double piercing | Capsule pierced twice before actuation |
| 4 | Shake prior to use | Device shaken (one up-and-down movement) before and after capsule piercing |
| 5 | Maintain the piercing button | Not releasing the piercing button during actuation |
| 6 | Incorrect inhaler position | DUSA positioned vertically (90° and -90°) during actuation |
Table 2: Reproduced in vivo handling errors and in vitro corresponding parameters.
The results of the simulations are summarised in Figure 1. A significant increase of the ED can be observed with increasing flow rate. Varying the volume of air drawn in the device from 2 L to 4 L does not significantly impact in vitro performance. However, tests performed at 10 L/min have shown a dramatic fall of the DPI performance (<0.4 μg ED, not shown in Figure 1). These data are illustrative of the stable performance of DPIs once critical flow of the device has been reached but also underline the critical need to monitor seriously ill patients’ and children’s inhalation strength when using DPIs. This aligns with the hypothesis made in Selestini’s study that DPIs were less frequently prescribed to patients with severe obstruction because of physicians’ fear that such patients would be unable to generate the inspiratory flow rate required for effective aerosolisation.
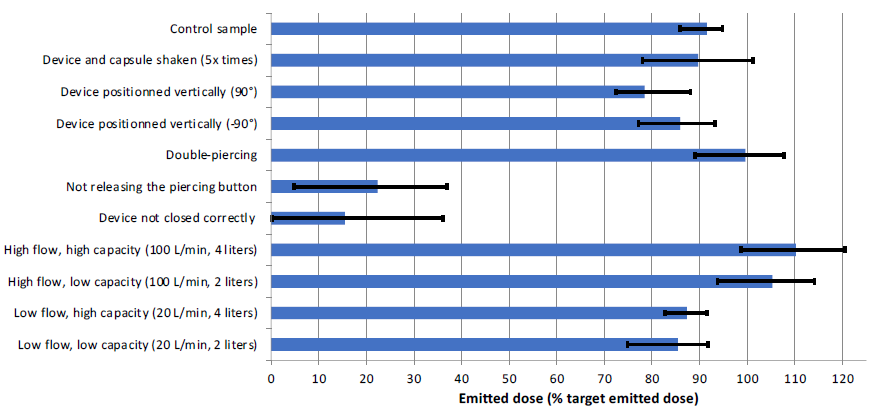
Figure 1: Impact of various handling and breathing errors on the performance of HandiHaler.
Among the different handling-specific errors that were reproduced, “not releasing the pressed piercing button” and “device not closed correctly” had the most significant impact on the delivered dose. The ED of these two handling errors was reducedto 22.4% (p<0.01) and 15.5% (p<0.01) of the TDD, respectively. This represents a drastic decrease that could negatively impact the efficiency of the patient’s treatment, considering that these handling errors are made by up to 45% of DPI users (up to 25% for HandiHaler).5 The ED obtained from an upright (+90°) position of the device also shows a 10% (p=0.01) decrease in the ED, while shaking of the loaded device before use (five up-and-down movements) and downward (-90°) actuations did not yield significantly different results from the control sample (p>0.05).
While the ED obtained from the control and the shaken device were comparable, the effect of the capsule being shaken prior to and after piercing led to a significant difference in the fine particle dose (FPD) delivered by the device, as summarised in Figure 2. The FPD decreased after a single up-and-down movement by 15% (p=0.05) prior to capsule piercing and by 28% (p<0.01) after capsule piercing. The significant decrease in the number of fine particles below 5 μm observed after a single up-and-down movement potentially highlights the role played by electrostatics when DPIs are erroneously manipulated and further shows the importance of controlling both the performance of the total dose delivered and the fine particle dose delivered to the lungs in vitro .
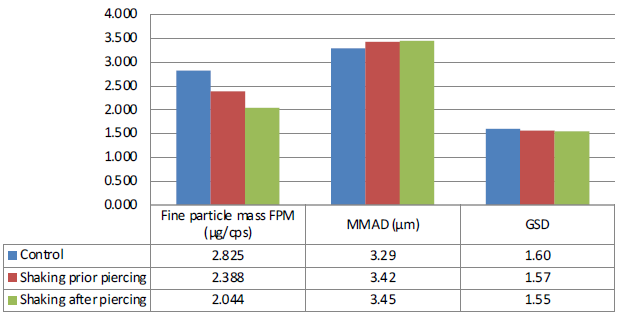
Figure 2: APSD performance of HandiHaler with a shaken capsule (one up-and-down movement) prior to and after piercing.
Overall, the results of thorough in vitro simulation of handling errors as part of a well-designed PCS can show the potential impact of patient non-adherence to the handling instructions presented in DPI leaflets. Although proper breathing techniques are important to achieve accurate and reproducible delivered doses, failure to meet specific items on the DPI handling checklist can lead to a drastic decrease in device performance, potentially affecting the chances of success of the treatment. Applicants or market authorisation holders should consider expanding the design space of in vitro testing during PCSs to cover all – or at least the most relevant – manipulation errors, as well as various respiratory capacities to obtain an accurate assessment of the impact of HF on device performance.
Usage data from HF and clinical studies allows for further assessment of the likelihood of non-adherence or manipulation errors. An evidence-based correlation of such data on likelihood and the potential impact of handling errors and variations via a risk impact matrix, as illustrated in Table 3, could drive a thorough understanding of associated risks for therapeutic effectiveness. Identifying the most critical device-handling steps can inform better instruction for patients and prescribing physicians on key handling errors, facilitate effective patient training and, ultimately, improve therapy outcomes.
| Risk matrix | Impact on device performance | ||||
| Minor | Moderate | Major | Critical | ||
| 0–25% | 26–50% | 51–75% | 76–100% | ||
| Likelihood of occurence | 76–100% | 3 | 4 | 4 | 5 |
| 51–75% | 2 | 3 | 4 | 4 | |
| 26–50% | 2 | 2 | 3 | 4 | |
| 0–25% | 1 | 2 | 2 | 3 | |
Table 3: Handling error risk matrix.
REFERENCES
- Price D et al, “Inhaler Errors in the CRITIKAL Study: Type, Frequency, and Association with Asthma Outcomes”. JACI: In Practice, 2017, Vol 5(4), pp 1071–1081.
- “Draft Guidance for Industry and FDA Staff: Human Factors Studies and Related Clinical Study Considerations in Combination Product Design and Development”. US FDA, Feb 2016.
- Sestini P et al, “Prescription Bias and Factors Associated with. Improper Use of Inhalers”. J Aerosol Med, 2006, Vol 19(2), pp 127–136.
- Van Der Palen J et al, “Comparison of the Diskus inhaler and the Handihaler regarding preference and ease of use”. J Aerosol Med, 2007, Vol 20(1), pp 38–44.
- Lavorini F et al, “Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD”. Respir Med, 2008, Vol 102(4), pp 593–604.

