To Issue 140
Citation: Authesserre C, Corne F, “An Airless Spray Nozzle to Produce a Fine and Soft Mist”. ONdrugDelivery, Issue 140 (Nov 2022), pp 49–52.
Claire Authesserre and Florian Corne discuss the current challenges faced by the topical spray-based delivery of therapeutics and how EVEON’s Ad-Mist™ spray nozzle overcomes these challenges to harness the potential of this delivery route.
SPRAY DELIVERY AS A NEW ROUTE OF ADMINISTRATION
Over the past decades, drug delivery has evolved and new routes of administration have emerged. When one thinks of drug administration, one usually thinks of swallowing pills (oral administration) or getting an injection (parenteral administration). However, drugs can also be delivered via other routes, such as the enteral route through tubing in the gastro-intestinal tract, inhalation to the lungs via the mouth or even the topical route via the skin (cutaneous, transdermal) or a mucosa (intranasal, buccal, auricular, ophthalmic, etc).
“As the biologic and controlled-release formulation markets grow,
there is a huge need to overcome high-viscosity formulation challenges with specific spray devices.”
The choice of delivery route for a drug depends not only on the site of desired action, but also on its properties – its absorption by the body and the environment in which it will be administered. Compared with the conventional oral and parenteral routes, it is claimed that topical administration offers the advantages of being non-invasive and avoiding first-pass metabolism in the liver by enabling the drug to enter directly into the circulatory system. This is an advantage when rapid drug absorption and onset of action are desired.
Depending on the route of administration, the formulation and the dosage, a delivery device may be needed to properly administer the drug. Over many years, a variety of spray devices have been developed, such as nasal or buccal sprays, inhalation systems and even spray-on bandage devices. Indeed, spray- or mist-based delivery presents many advantages over conventional methods:
- Delivery to areas with limited access
- Delivery in any patient posture, in particular, in any patient’s head position for intranasal, buccal or auricular administration
- Overdose-free and high surface coverage – precise delivery of a small dose to a large surface area – increasing the drug’s bioavailability
- Safety – non-contact, non-invasive and needle-free delivery
- Convenient and easy to use.
CHALLENGES IN SPRAY DELIVERY
Atomisation of Highly Viscous Formulations
High-viscosity formulations have become increasingly prevalent over the last few years in the pharmaceutical industry. This new paradigm could be attributed to several key factors. The first one is the significant growth of biologics. Monoclonal antibodies (mAbs) are expected to reach combined global sales of US$150 billion (£134 billion) by 2022. Biosimilars have also entered the market, creating a highly competitive landscape.
The second key factor is the increasing development of sustained/controlled release formulations. These long-acting formulations are intended to reduce the required dosing frequency of a therapy, improving patient compliance and reducing impact on healthcare costs. There are several technical strategies that can be used to develop a controlled release formulation, but the main challenge is that they could be difficult to deliver. For example, they could include high molecular weight polymers or other vehicles, increasing the viscosity of the entire formulation. As the biologic and controlled-release formulation markets grow, there is a huge need to overcome high-viscosity formulation challenges with specific spray devices.
Airless and Propellant-Free Spray Delivery
The use of compressed gas or propellant can facilitate the atomisation of a liquid, especially when it is highly viscous. However, the contact of the drug with air can lead to oxidation of the drug and can increase the risk of microbial contamination. Therefore, the challenge is to develop an airless spray nozzle for drug preservation and sterility, and propellant-free devices to provide a more eco-friendly alternative to existing products.
Fine and Soft Mist
For pharmaceutical applications, delivering a fine mist improves the efficacy of the delivered formulation by improving the topical absorption of the drug by the tissue. Additionally, a soft mist is important for minimising the pain or discomfort felt by the patient. In industries other than pharmaceuticals, such as cosmetics, fine and soft mist delivery is required to improve the user experience.
Figure 1: Ad-Mist nozzle.
DEVELOPMENT OF A SPRAY NOZZLE
EVEON has designed and patented a spray nozzle, the Ad-Mist™ nozzle (Figure 1), requiring neither air nor propellant, that overcomes these spray delivery challenges. Different versions of the nozzle have been designed, based on the same technology, to adapt to the requirements in terms of flow rate, droplet size, inlet pressure and spray angle, among others.
Viscosity & Viscoelastic Properties
The Ad-Mist nozzle can adapt to a large range of viscosities, including highly viscous formulations. For example, the Ad-Mist nozzle is capable of spraying a gel with a viscosity of 8,000 Pa·s at 0.1s-1 with a shear-thinning index of <0.1. However, it is critical that the inherent properties of the formulation, such as the viscoelasticity, are not altered by the spraying mechanism.
To evaluate the impact of the spray on the gel structure, viscoelastic properties of the gel were characterised on a rheometer and a comparison delivery between the gel deposited on the rheometer plate with a cannula and the gel deposited by the spray device (Figure 2). With the spray, a small relaxing time can be observed but, in less than 200 s, the gel regains its original rheological properties – equivalent to standard, non-spray, cannula deposition. This demonstrates that the Ad-Mist nozzle developed by EVEON enables proper conservation of the viscoelasticity properties.
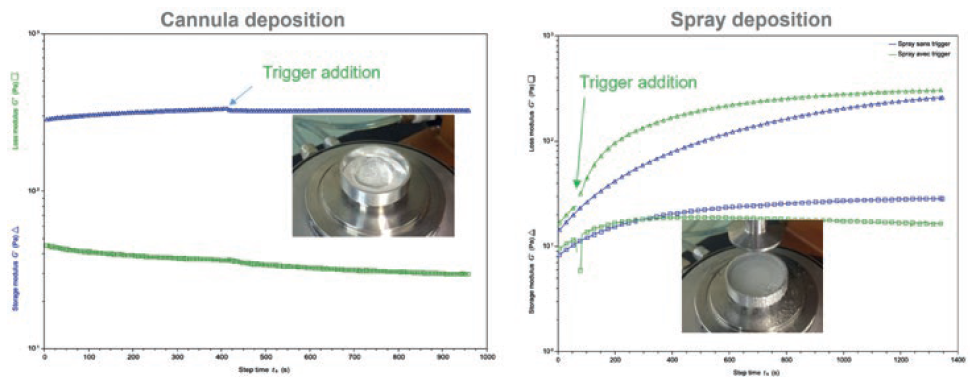
Figure 2: Conservation of visco-elastic properties after spraying with the Ad-Mist nozzle.
“Designed for a precise dose administration, EVEON’s Intuity® Spray is capable of delivering doses from 15 μL to several millilitres with 1 µL accuracy.”
Particle Size
The droplet size of the mist produced by the Ad-Mist nozzle has been characterised using a laser diffraction system (Spraytec – Malvern Panalytical) with several formulations having viscosities from 1 to 14 cP. The measurement was performed at 2 cm and 15 cm from the nozzle outlet; Figure 3 shows the results obtained at 2 cm. Whatever the viscosity, the mean droplet size was around 20 μm, with 90% of the droplets below 45 μm in diameter. Fewer than 10% of droplets were below 10 μm in diameter, limiting the amount of mist reaching the lungs. The mean droplet size measured at 15 cm was around 40 μm.
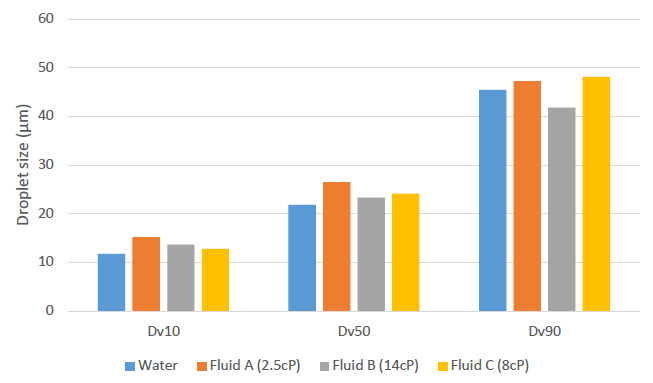
Figure 3: Droplet size of the mist produced by the Ad-Mist nozzle.
Surface Covering
The Ad-Mist nozzle is capable of delivering a homogenous distribution of droplets to a surface. The area covered by the mist directly depends on the spray plume geometry, which can be tailored with the nozzle fluid path geometry and flow rate between 25° and 60°. For example, a spot size of 1 cm is obtained with water at a distance of 2 cm from the surface. With a continuous delivery using a flow rate of 0.15 mL/s, the Ad-Mist nozzle can cover a 100 cm2 area within 15 s.
“EVEON’s Ad-Mist spray nozzle is a versatile platform that can be integrated in a broad range of devices and adapted to the required use gesture and user experience.”
Low-Impact Pressure
In collaboration with its sister-company ALPAO (Montbonnot-Saint-Martin, France), EVEON was able to measure the impact pressure generated by the Ad-Mist nozzle thanks to an optical system based on ALPAO’s deformable mirror core technology. During a topical administration, Ad-Mist’s droplets impact a surface at 2 N/m² when sprayed 2 cm away from the administration site. This is comparable with the impact of a fly landing on a stamp. The low-impact pressure generated by the Ad-Mist nozzle provides significant potential for administration to strongly innerved or sensitive areas.
DEVELOPMENT OF SPRAY DEVICES
EVEON’s Ad-Mist spray nozzle is a versatile platform that can be integrated in a broad range of devices and adapted to the required use gesture and user experience – usability being a key factor for device acceptability on the part of the user. EVEON is used to working with ergonomists and designers to take human factors into account, right from the first steps of device design, to ensure that the device design is appropriate to the target user population and easy to use.
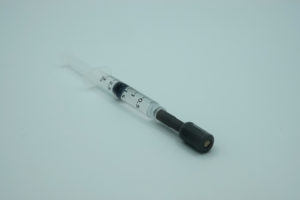
Figure 4: Ad-Mist nozzle for syringe – easy mist delivery.
The Ad-Mist nozzle can be used with a standard syringe thanks to a Luer connection for a continuous and manual administration of the drug (Figure 4). The nozzle can be connected to a syringe as simply as a needle would be. It is the easiest way to produce a spray when there is no specific dosage requirement and the viscosity allows for a reasonable force to be applied to the syringe plunger.
If precise control of the administration and spray quality are required, this spray nozzle can also be integrated into the Intuity® Spray platform (Figure 5). Due to its unique electronic and fluidic features, this tailored, easy-to-use medical device offers controlled flow rates and dosages. The mist can be delivered with a highly controlled flow rate between 0.1 and 1 mL/s, as either a continuous or a dose delivery due to the integration of a micropump specifically developed by EVEON.
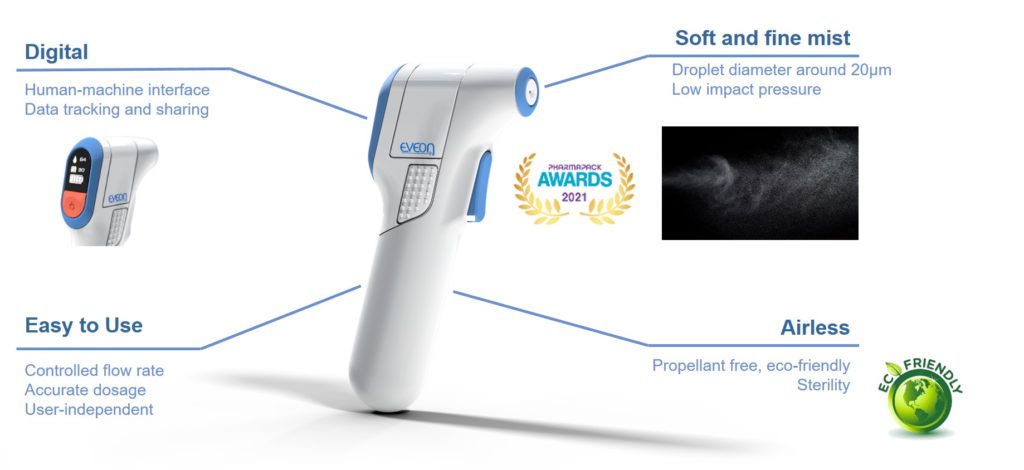
Figure 5: Intuity Spray – highly controlled mist delivery.
Designed for a precise dose administration, EVEON’s Intuity® Spray is capable of delivering doses from 15 μL to several millilitres with 1 μL accuracy. The spray quality is highly reproducible; the standard deviation of the mean droplet size between two sprays is less than 1 μm. Therefore, by using Intuity® Spray in combination with the Ad-Mist nozzle, the mist administration can be standardised.
CONCLUSION
Administering drugs in the form of a spray, particularly for topical administration to the skin or mucous membranes, is a major trend in the pharmaceutical industry. Sustained market growth is expected, with an annual growth rate of +7.3% between now and 2026 for mucosal administrations. Compared with parenteral routes, sprays offer undeniable advantages; as an easy to use, non-invasive medical device, they can improve therapeutic adherence, particularly for self-administration.
EVEON is the right partner to build custom airless spray nozzles for gels, and highly viscous and non-viscous solutions, suitable for standard primary containers and adapted to the intended usage.

