To Issue 140
Citation: “Advancing the Science: Exploring New Alternative In Vitro Tests to Help Demonstrate Bioequivalence in Inhalation Products”. ONdrugDelivery, Issue 140 (Nov 2022), pp 82–86.
Proveris Scientific looks at recent advances in in vitro testing in inhalation products to demonstrate bioequivalence and discusses the company’s INVIDA platform that replicates human usage of the device.
REGULATORY LANDSCAPE
The first pressurised metered dose inhaler (pMDI) was invented by Charles Thiel and developed by a small team at Riker Laboratories (MN, US) back in 1956 to treat asthma.1 Since then, pMDIs have become the preferred delivery system for the treatment of lung diseases, including chronic obstructive pulmonary disease, emphysema and chronic bronchitis, because “small doses of drug are delivered directly to the site of action, leading to an onset of actions and a low incident of side effects”.2 In addition, innovation in pMDI development has led to newer delivery systems, such as dry powder inhalers (DPIs) and soft mist inhalers (SMIs), each with their own unique delivery mechanisms and benefits for patient usage and formulation compatibility (Figure 1). Furthermore, research is now underway to apply the delivery mechanisms to other therapeutic areas, such as vaccine delivery and biologics.

Figure 1: Delivery systems for inhaled drug products.
With the long history of generic drug approvals in parenteral and oral dosage forms, it might be expected that inhaled therapies would easily follow suit. However, due to the complexity of the relationships between device performance, formulation and patient usage, this has not been the case. Currently there are only a small handful of approved generic inhaled therapies in the US.
With the approval of the first generic version of ProAir HFA (albuterol sulfate) from Teva (Tel Aviv, Israel) in February 2020, US FDA commissioner Stephen Hahn stated:
“Proveris has built into the PFV measurement the ability to calculate the spray duration of the product using the average image-intensity data for each spray.”
“Metered dose inhalers like these are known as complex generics, which are traditionally harder to copy because of their complex formulation or mode of delivery. As a result, many complex drugs lack generic alternatives even after patents and exclusivities no longer block generic approval. Supporting development and approval of generic copies of these complex medicines so that these products can get to patients has been a major focus of our efforts to improve competition and access and to lower drug prices. Getting more generic copies of complex drugs to the market is a key priority for how we’ll help bring new savings to consumers.”3
In accordance with this statement, there has been a dramatic shift in the regulatory landscape, allowing for more of a defined roadmap to generic approvals.
In 2007, the FDA first introduced the idea of Product-Specific Guidance (PSG), which provides specific recommendations on individual drug products as a framework to guide developers towards successful market approval. In 2017, with the release of the Generic Drug User Fee Amendments, it increased the commitment, issuing PSGs for ~90% of the non-complex new chemical entities (NCEs) that were approved on or after October 1, 2017. In addition, it offered a commitment to provide future PSGs for complex products as scientific recommendations become available. As of June 1, 2021, approximately 1,900 PSGs have been released.
For inhalation products, typically these PSGs have listed several commonly accepted in vitro tests that data must be provided for in a submission package for an ANDA.4 Traditionally, these have included tests such as:
- Single actuation content through container life
- Droplet size distribution by laser diffraction
- Aerodynamic particle size distribution by cascade impaction
- Drug particle size by microscopy
- Spray pattern
- Plume geometry
- Priming and repriming.
However, as recently as May 2019, the FDA has shown a commitment to expanding this list of tests and has tasked the industry with exploring new alternative approaches to the comparative clinical endpoint bioequivalence study.5 Some of these new tests that Proveris Scientific focuses on include characterisation of spray-velocity profiles, spray duration, propellant evaporation rates and the use of more human-realistic mouth-throat models and breathing profiles for drug deposition tests.

Figure 2: SprayVIEW measurement system SFpMDI configuration, Vereo® SFMDx and NSx automated actuators – the technology used to perform PFV, spray duration and evaporation rate measurements for pMDIs and SMIs.
PFV & SPRAY DURATION ACHIEVED WITH THE SPRAYVIEW® MEASUREMENT SYSTEM
Proveris Scientific’s SprayVIEW measurement system has long been the industry standard for measuring and analysing the spray pattern and plume geometry of spray and aerosol devices. With the addition of spray velocity and evaporation rate to the list of proposed in vitro tests, the technology has been adapted to perform these measurements on the same common platform. For plume front velocity (PFV), the set-up can be achieved using a standard method set-up for a plume-geometry measurement for both the SprayVIEW for pressurised metered dose inhalers (SFpMDIs) and for oral sprays (OSP) configurations shown in Figure 2.
PFV is currently available as a contract service through Proveris Laboratories and can be performed on a customer’s SprayVIEW measurement system with the recent release of Viota® software at Revision 9.0. The measurement itself is achieved by using an image-processing algorithm to track the leading edge of the plume as it is emitted from the mouthpiece of an inhaler using image intensity data. For each image that is taken with the camera, the system will produce a series of data points consisting of distance and time components until the plume has exited the field of view (Figure 3a). Therefore, to maximise the amount of data collected per spray, it is important to adjust the method to maximise the field of view. Once the raw data is collected, a curve fit equation can be applied to the data (Figure 3b). This allows the user to interpolate and solve for velocity at any distance or time they wish. This new functionality allows users to easily generate PFV data according to the methodology listed in the November 2020 Tiotropium Bromide PSG for SMIs.6
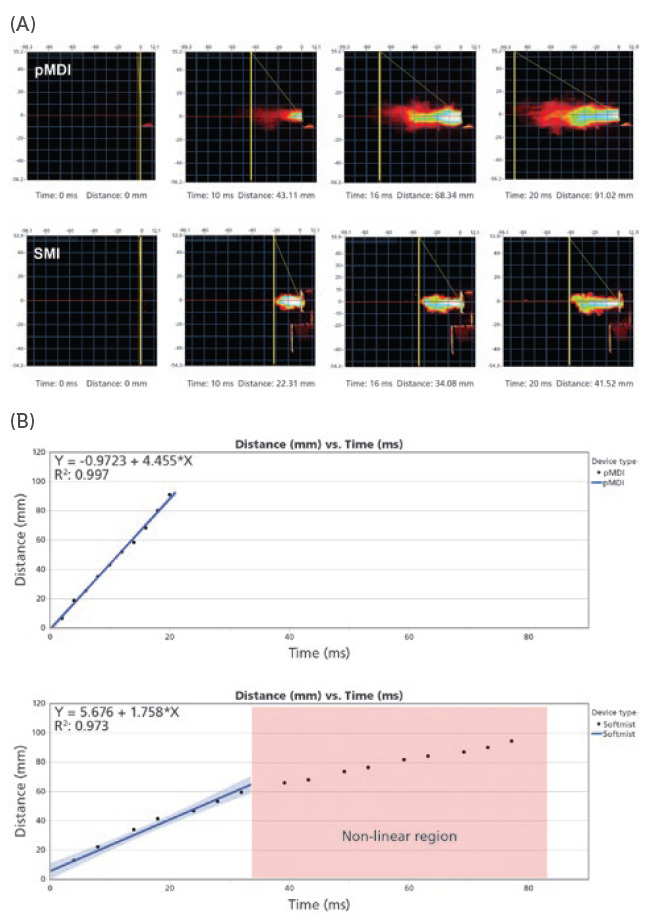
Figure 3: (A) Image captures taken with the SprayVIEW measurement system camera produce a series of data points consisting of distance and time components until the plume has left the field of view. (B) Graph plots of the distance versus time data points based on calibrated, time-synchronised plume sequence analysis.
“The INVIDA platform has the potential to accelerate product development and approvals by providing the information necessary for predictive clinical outcomes and bioequivalence.”
In addition, Proveris Scientific has built into the PFV measurement the ability to calculate the spray duration of the product using the average image-intensity data for each spray. The system uses an automatic algorithm to detect the start and stop times for the spray based on the image intensity (Figure 4). Alternatively, the user can switch to manual mode and drag the cursors to set the duration before saving the measurement.
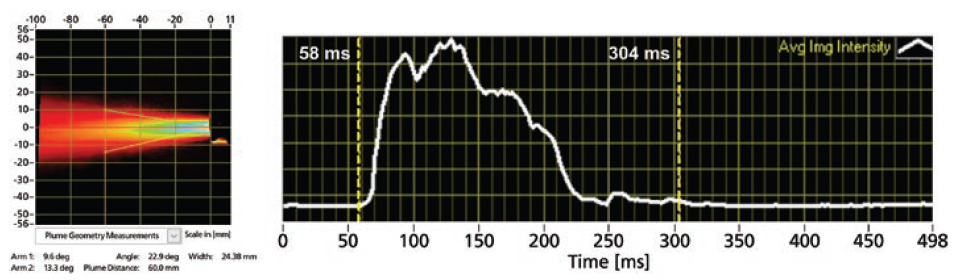
Figure 4: An example of time-averaged plume results (left) with time-synchronised intensity profile (right, white curve) indicating the spray duration (58–304 ms).
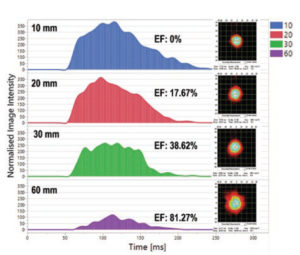
Figure 5: The area under the curve (AUC) indicates the drug mass at a certain distance from the pMDI mouthpiece: the AUC decreases as the spray aerosol moves further away from the mouthpiece because of drug mass loss due to evaporation.
EVAPORATION RATE
Evaporation rate studies are currently offered exclusively through Proveris Laboratories as a contract service. The evaporation fraction represents the relative amount of aerosol that has evaporated at specified distances from the mouthpiece of the product. The evaporation rate is simply defined as the rate of change in the evaporation fraction with respect to time.
The methodology includes taking spray pattern images at defined distances from the mouthpieces (usually 10, 20, 30 and 60 mm). By calculating the area under the curve of the image intensity picked up by the SprayVIEW’s high-speed camera, Proveris Laboratories can correlate through proprietary algorithms how much of the bulk mass of the spray has evaporated (Figure 5). This technique has proven highly informative for several customers to support their development process.
PROVERIS’S INVIDATM PLATFORM
The INVIDA platform represents a new paradigm for in vitro analysis of aerosol products. By incorporating human-realistic mouth-throat models and a novel breathing simulator capable of creating complex programmable breathing profiles, both quantitative and qualitative insights into a product’s performance are provided. The INVIDA platform has the potential to accelerate product development and approvals by providing the information necessary for predictive clinical outcomes and bioequivalence. This innovative technology measures the aerosol dispersion and regional deposition of inhaled drugs under simulated human-realistic conditions, enabling scientists to better understand their product’s performance through visual mapping within the respiratory tract and quantitative measurements of drug deposition that are more predictive of in vivo performance.
The platform consists of human-realistic mouth-throat models that are capable of being swapped out for other geometries. A Vereo automated actuator is mated to the mouth to fire the drug under controlled and repeatable parameters. Downstream from the throat, there is an optical flow cell where the flow of the drug can be visualised using similar technology to the SprayVIEW system. This allows for qualitative visualisation of the aerosolisation characteristics, providing key insight into its performance throughout the entire breathing cycle. This aspect of the system can be valuable as a comparative tool when evaluating different formulations or device types.
Downstream from the flow cell is a filter, or series of filters depending on the configuration, allowing for capture of the drug product. In addition, all pieces of the mouth-throat assembly are specially coated to promote drug adherence and mimic conditions inside the lung. Humidity and temperature control of the INVIDA system replicate the high humidity conditions within the human respiratory tract. All parts in the assembly are easily disassembled and can be assayed separately to obtain a full mass balance for quantitative deposition of the API(s) in question. This data provides the development team with localised deposition of the active drug under human-realistic breathing conditions to better assess performance and complement approaches for in vitro-in vivo correlations. The INVIDA system can achieve fully programmable complex profiles, including flexibility to control inhalation, hold times and exhalation, offering the ability to perform a wide range of tests. In addition, there is capability to simulate disease states including left/right lung imbalances.
When experiments are performed early in the development cycle, INVIDA can offer valuable insight into the performance of a product, allowing critical decisions to be made that could save time and money over the course of a project (Figure 6). Compared with cascade impaction, the platform offers significant potential in terms of pharmacokinetics correlation due to the human-realistic nature of the set-up.
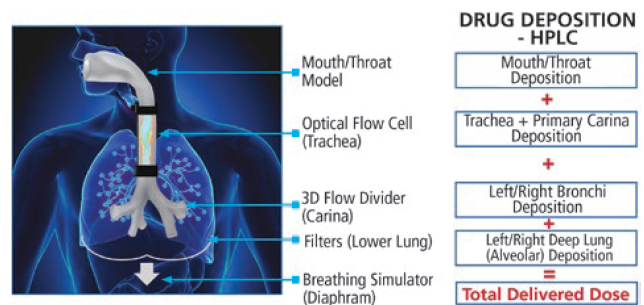
Figure 6: Schematic of Proveris Scientific’s INVIDA platform.
CONCLUSION
The performance of orally inhaled and nasal drug products is affected by a combination of formulation, device and patient usage. The Proveris Scientific and Proveris Laboratories approach closely follows the approach recommended by the FDA and can save both time and resources while accelerating the time to market of these products. Alternative in vitro tests that are human realistic can enable companies to make data-driven decisions and expedite product development and approval while saving time and resources. The Proveris Scientific in vitro testing platform INVIDA is built to replicate human usage of the product (in terms of breathing profile, respiratory geometry and environment), while minimising the gap between in vitro and in vivo performance to predict true product performance.
REFERENCES
- Melling L, “Inventing the MDI: A History in Modern Inhalation Therapy, 3M’s Charlie Thiel offers insight into the pressurized metered-dose inhaler”. Contract Pharma, April 19, 2017.
- Newman SP “Inhaler treatment options in COPD”. Eur Respir Rev, 2005, Vol 14, pp 102–108.
- “FDA approves first generic of ProAir HFA, Agency Supports Development of Complex Generic Drugs to Improve Competition and Access to More Affordable Medicines”. Press Release, US FDA, Feb 24, 2020.
- “Guidance for Industry: Bioavailability and Bioequivalence Studies for Nasal Aerosols and Nasal Sprays for Local Action”. US FDA, Apr 2003.
- “Draft Guidance on Beclomethasone Dipropionate”. US FDA, May 2019.
- “Draft Guidance on Tiotropium Bromide”. US FDA, Nov 2020.

