To Issue 149
Citation: Hudson-Farmer K, Hall S, “Reimagining Autoinjector Connectivity.” ONdrugDelivery, Issue 149 (Jun 2023), pp 44–46.
Kate Hudson-Farmer and Simon Hall discuss a new smartphone-based connectivity solution for autoinjectors that requires no additional hardware and works with most autoinjectors and smartphones.
The rise in treating chronic diseases with biologics over the past 20 years has been significant. This has led to an increase in demand for self-administration of subcutaneous therapies – and a corresponding growth in the development and use of autoinjectors. In recent years, digital connectivity for autoinjectors and other self-administration devices has been seen as the future for improving patient engagement and outcomes.
“An alternative solution that addresses existing market challenges comes from a completely different way of connecting disposable mechanical devices.”
However, despite this move towards connecting autoinjectors, many organisations fail to create a business case for such devices due to current constraints and risks. This leads to patients missing out on the benefits of a connected drug delivery environment, healthcare professionals missing out on insights into how patients are coping, and a missed learning opportunity for pharmaceutical and medical device companies and the wider science community.
There is a real opportunity for more sustainable, cost-effective, data-rich and device-agnostic connected solutions that support and liberate the pharmaceutical and healthcare sectors from current limitations but also empower and improve quality of life for patients and their carers.
CURRENT AUTOINJECTORS AND CONNECTING THEM
Autoinjectors were primarily devised as devices that help a patient self-administer a prefilled syringe safely and easily. To address ease of use, manufacturing, and industry models, the predominant autoinjector is a disposable, single-use, mechanical, two-step autoinjector. These autoinjectors provide relatively simple feedback to the patient in the form of visual signalling, mainly through the window to view the plunger moving, in addition to mechanical audible clicks to signal injection progress.
The majority of autoinjectors are composed of plastic and metal. In more recent years, connectivity has been achieved by adding on hardware-specific plastic and electronic components that capture data. The purpose of digital connectivity of autoinjectors is to help the patient use the device, record they have taken the medication, remind them to take it, store a history of medication use and share information. These factors are aimed at ensuring the medication is taken properly and in line with the treatment and dosing regime in a non-clinical setting, primarily in the home environment.
“Where current connected autoinjector solutions are expensive, device specific, introduce more plastic to the environment and only provide limited data, ARinject aims to achieve the exact opposite.”
Simplifying Connectivity, the Supply Chain and the Patient Experience
An alternative solution that addresses existing market challenges comes from a completely different way of connecting disposable mechanical devices. Developed by PA Consulting, ARinject™ is a smartphone-based connectivity solution for autoinjectors that requires no additional hardware and works with any type of autoinjector and smartphone. Where current connected autoinjector solutions are expensive, device specific, introduce more plastic to the environment and only provide limited data, ARinject aims to achieve the exact opposite (Table 1).
| Existing Connected Devices | ARinject | |
| Cost |
Add-on devices are expensive to develop – each add-on is specific to a type of disposable autoinjector. Additional manufacturing, storage and distribution is required on top of the disposable autoinjector. |
Involves software development only, meaning less cost than hardware and associated software development; no hardware manufacturing, storage and distribution costs. Works with existing commercial and research products and with most autoinjectors without modification. |
| Time | Regulatory development and manufacturing required to get approved and on market takes several years. | Faster development than hardware solutions; no manufacturing scale up and down; no logistics or component sourcing required. |
| Sustainability | Add-on devices are composed of plastic, electronics and metal so they deplete natural resources and are not easy to recycle. | Software based so no additional plastic, metal and electronics components to make or dispose of. |
| Usability | Adding a device to an autoinjector adds extra steps in the injection process. Patients have to store the add-on, remember where it is, add it to the injection device prior to injection, take it off and store it after injection and not accidently throw it away in the sharps bin with the disposable autoinjector. |
Only requires a smartphone, no add-on devices to learn how to use and store. Suitable for patients using multiple injectable products – |
| Evidence |
Building business cases and return on investment models for connected health solutions relies on data from market examples, which are currently few and far between due to the cost and time to bring them to market. Many companies have also been expected to provide returns based on reimbursement for such connected offerings. However, gaining reimbursement is, again, a difficult proposition as there needs to be a proven example of success first – for example, increased patient outcomes data, such as that pertaining to adherence. Current business models also rely on these solutions being used by large numbers of patients over long periods of time. |
Greater flexibility for patient numbers so more easily scalable to gather data to support market propositions. |
A patient-centric approach to home-based healthcare
ARinject uses sophisticated image processing, augmented reality techniques and machine learning to offer simple, real-time dosage guidance for patients and their carers. ARinject recognises the autoinjector type, label, cap on/off, autoinjector positioning on injection sites and dosing progression, and incorporates usage notifications and reminders. It also allows for front and rear camera use, eliminating the need to hold the phone, to enable two-handed injector use, if necessary.
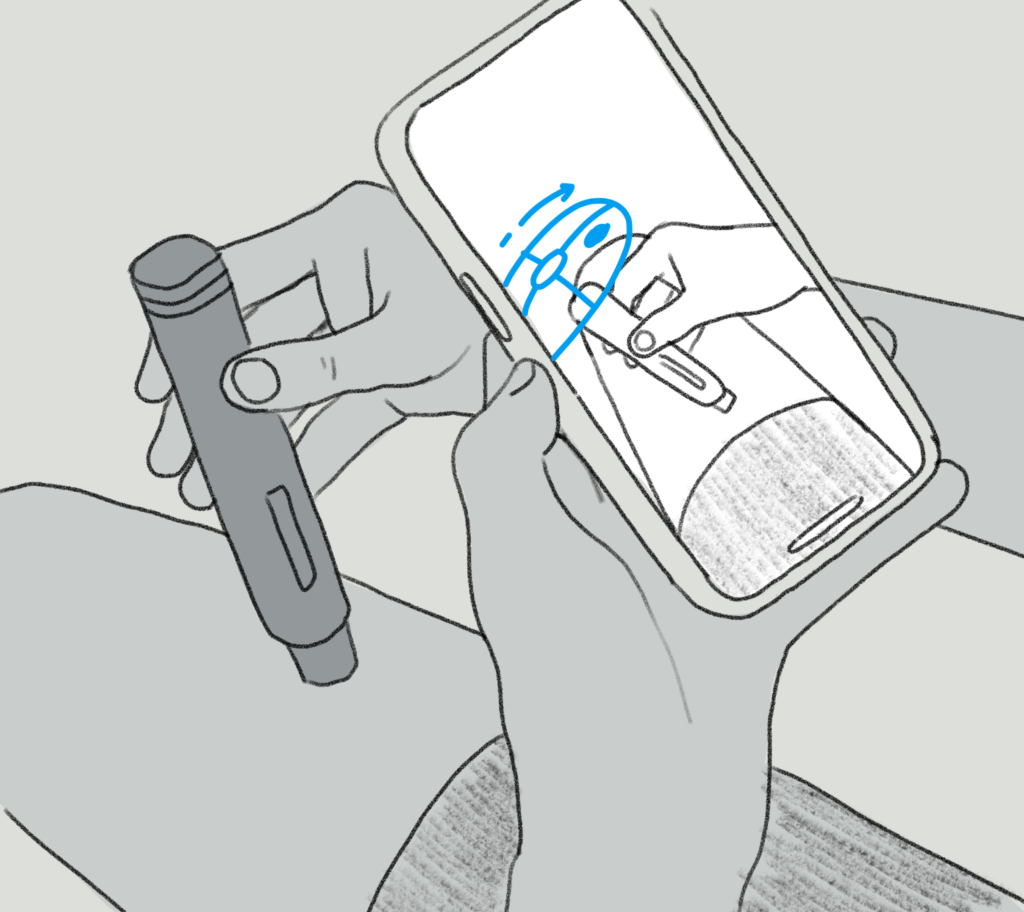
Figure 1: ARinject’s image processing captures injection and dosing information and increases patient confidence.
ARinject not only captures injection and dosing information to increase patient confidence that they have taken the dose at the right time and day, and records this for future reference, but it also helps guide them through the injection process in real time (Figure 1). This feature could provide both comfort and support to patients unsure of how to inject, in addition to carers gaining confidence – and provide support for more difficult dosing regimens, including during the early stage of a patient’s treatment or in clinical trials.
MULTIPLE USE CASES ACCELERATE OPPORTUNITY
ARinject is currently at proof-of-concept stage, with early user studies showing that people can successfully perform a demonstration injection using ARinject. As ARinject is a platform for development, any company with an autoinjector could connect to it and, because no modifications to the autoinjector are required, this approach could be developed for any autoinjectors – whether they are on the market in commercial use or in development. Use cases range, therefore, from clinical trials – to help guidance and training for autoinjector use – to on-market products where more training could help certain patient groups and adherence tracking, particularly with small patient groups needing greater support.
The versatility, lower cost and time, and low environmental impact of ARinject aims to revolutionise the way autoinjectors are connected, and the way training and guidance is offered to autoinjector users. By reimagining this market, PA Consulting aims to help open it up and make it faster, cheaper and available to the many rather than the few, so that the question of return on investment and business case are not as prohibitive. Everyone can then start to benefit from the data that comes from these approaches, and patients will have greater engagement opportunities and improved outcomes.
PA Consulting is looking to work with pharma or medical device partners to develop the ARinject in specific use cases for particular indications and autoinjectors. The company is open to discussions on how to proceed with such software developments to create customer specific variants.

