To Issue 175
Citation: Fleutsch G, “Interview: SHL Advantec – SHL Medical’s New Manufacturing Equipment Sub-Group”. ONdrugDelivery, Issue 175 (Jul 2025), pp 14–17.
Gilbert Fluetsch discusses the establishment of SHL Advantec and the foundation it has built as SHL Medical’s in-house equipment specialist. Mr Fluetsch also looks at the role of modular platforms in scaling production and how the company’s global network is designed to support the changing landscape of medical and pharma manufacturing.
Q To start with, can you give us a brief overview of SHL Advantec and its relations with SHL Medical, a well-established company in the drug delivery space?
A SHL Advantec is a global provider of high-precision moulds and advanced automation systems for the manufacturing, assembly, handling and testing of medical devices and other applications in the wider healthcare sector. Our two core areas of expertise – tooling and automation – originated from SHL Medical’s in-house manufacturing capabilities. More specifically, SHL Advantec was formed from the two departments that were responsible for designing and manufacturing the injection moulds, as well as the assembly and testing equipment, for over 90% of SHL Medical’s autoinjector and pen injector projects.
“SHL MEDICAL IS OFTEN CONSIDERED TO BE ONE OF THE PIONEERS OF THE AUTOINJECTOR SYSTEMS WE KNOW TODAY.”
SHL Medical is often considered to be one of the pioneers of the autoinjector systems we know today. Specifically, SHL’s DAI® autoinjector was one of the most popular autoinjectors on the market for years after its initial commercial launch, laying the foundation for modern self-injection treatment. What some might not realise is that the team now at SHL Advantec has been right there from the beginning of it – working behind the scenes on all of SHL’s bespoke and platform devices over the past 20 years. We were the ones designing the low-cavitation moulds, building simple test fixtures and assembly equipment – while, at the same time, pioneering some of the industry’s first testing methods. As early as 2002, we had begun developing a semi-automatic testing machine (SATM) for the DAI autoinjector (Figure 1). The first of its kind in the self-injection space, this SATM broke new ground by introducing automation to autoinjector testing.

Figure 1: An SATM developed for SHL Medical’s DAI® autoinjector, which saw its first commercial launch in 2006.
Today, we are still supporting pharma’s growth all the way through to high-volume production. In 2010, SHL Medical set another industry benchmark with the launch of the two-step Molly® autoinjector. When Molly evolved into a modular platform in 2021 – offering greater flexibility in both design and manufacturing – our tooling and automation experts were alongside that journey once again. We didn’t just develop the production equipment – we also brought in design-for-manufacturing expertise to ensure that the new platform could scale efficiently, adapt to multiple drug products and meet the evolving needs of pharma customers. These experiences gave us a deep understanding of what it takes to bring reliable, scalable manufacturing solutions to complex self-injection systems. And today, as SHL Advantec, we are carrying that know-how forwards and making our experience available across the medtech and pharma sectors to help more customers solve similar challenges.
“WHAT STOOD OUT WAS HOW CONSISTENTLY THOSE CUSTOMERS CAME BACK WITH POSITIVE FEEDBACK – NOT JUST ON THE PERFORMANCE OF OUR EQUIPMENT, BUT ALSO ON THE LEVEL OF SUPPORT AND SERVICES WE PROVIDED.”
Q What led to the decision to create SHL Advantec?
A That decision really came from the market itself. Over the years, we’ve had a lot of interactions with pharmaceutical companies and, in those conversations, we kept hearing the same thing – that our solutions were right up there with, if not better than, what was available commercially. In fact, some customers even started asking if they could use our equipment for their own production lines, outside of SHL Medical’s device programmes. And, the truth is, we were partially doing this already. We have built and delivered final assembly and device testing equipment directly to pharma or their CMO’s production sites in Asia, Europe and the US, so working directly with pharma wasn’t entirely new to us. What stood out was how consistently those customers came back with positive feedback – not just on the performance of our equipment, but also on the level of support and services we provided. That confidence really made us take a step back and realise that there is a broader market for what we do, even beyond drug delivery devices. That insight is what ultimately led us to establish SHL Advantec.
Q SHL Advantec brings together several businesses, including LCA Automation, SMC Mould Innovation and Superior Tooling, each with its own pedigree. What does bringing them together under one roof unlock?
A Each of these three companies bring decades of experience with them – going back to 1925 in the case of SMC Mould Innovation – so there’s a lot of deep expertise there. They’ve all worked with top pharma and medtech companies, but also operated in other demanding industries such as aerospace, construction and even watchmaking. That diversity really adds to SHL Advantec’s strength.
Take LCA Automation for example. LCA has done a lot in the automotive space, which shares the same focus on end-user safety as medical devices, but moves at a much faster pace. Pair that with SHL Advantec’s strong foundation in medical quality systems and you start to see the benefit of bringing everyone under one roof. We’re already combining that experience, especially in project and quality management, to align our operations across sites. That means more efficient design and development processes, smoother execution and, ultimately, faster, more responsive support for our customers.
Together with these sub-brands, we are building a comprehensive global network across three continents, which lets us optimise supply chains for our customers. What’s more, each of these sub-brands can now serve the others’ customers across industries. So, whether it’s aerospace, pharma, automotive or electronics, the combination of our expertise makes us stronger and more capable of meeting diverse customer needs.
Q How does establishing SHL Advantec as a new sub-group enhance SHL Medical’s overall offering?
A Again, it ties back to the global supply strategy. SHL Medical is significantly expanding its manufacturing footprint in the US and Europe. Just this April, it opened its new manufacturing site in North Charleston, South Carolina – its second in the US, alongside the final assembly facility in Deerfield Beach, Florida. On top of that, at least two more facilities are underway, one in Zug, Switzerland, and one in Taoyuan, Taiwan.
With SHL Advantec now operating across the same three continents – with some of our sites just a few hours away from SHL Medical’s facilities – we’re in a strong position to support SHL Medical’s global footprint. That proximity means faster response times and smoother integration between tooling, automation and final assembly – wherever production is happening. In turn, this helps SHL Medical deliver on its promise to its pharma customers with even greater speed and agility.
It’s also worth noting that SHL Medical’s device technologies are constantly evolving alongside the latest advances in drug development. From the classic DAI autoinjector and the modular Molly platform technology, to the most recent Elexy™ electromechanical autoinjector, SHL Medical has consistently been ahead of the curve in innovating drug delivery devices. With SHL Advantec now operating as an autonomous entity, SHL Medical has the opportunity to increase its focus on pioneering the next generation of self-injection solutions. And as always, the devices will be supported by cutting-edge equipment solutions by SHL Advantec – now strengthened by the combined expertise of our sub-brands.
Q How does SHL Advantec support the scaling journey, from small-volume clinical or pilot builds through to high-volume commercial production?
A The short answer is “platforms”. The longer answer is a bit more nuanced. Much like SHL Medical’s autoinjectors, we originally started with highly customised moulds and automation systems tailored to each project. But over time – and through a lot of first-hand experience – we’ve learned that scaling isn’t always linear. A product might start with small clinical batches, then suddenly ramp up if the treatment takes off. Or, as another example, one device design might need to serve multiple drug products, each with slightly different variations in its technical specifications.
From a device perspective, SHL Medical responded with the modular Molly technology by adding an extra level of design and production flexibility to an already powerful platform. From a manufacturing perspective, we also applied modularity into our equipment strategy. We’ve built up the ability to scale from early clinical runs all the way to full commercial production, without having to reinvent the wheel each time. A good example is our modular mould strategy. These moulds follow a consistent design language but vary in size and cavity configuration (Figure 2). With interchangeable cores, cavities and inserts, we can quickly adapt to different production needs – especially for projects with complex geometries or evolving requirements. This approach cuts down on lead times, lowers costs and adds a layer of manufacturing agility that is essential in today’s fast-moving medical landscape.
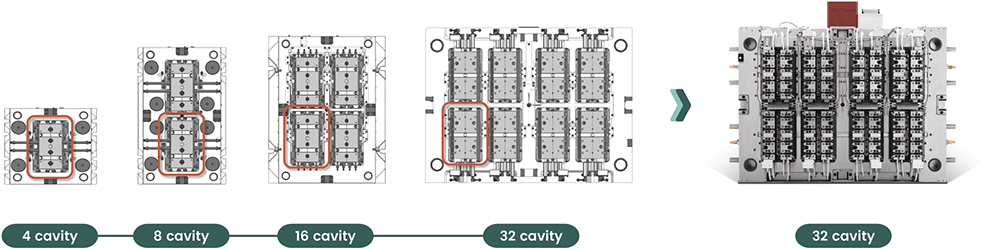
Figure 2: Left: Modular mould inserts with identical stack units in varying configurations. Right: Complete production mould for an autoinjector rear cap.
We’ve already seen this approach succeed in real-world scenarios. For example, we supported the scale-up of a Molly-based autoinjector used to treat cardiometabolic diseases that was originally forecasted for much lower volumes. As demand grew, we ramped up production by delivering several high-cavitation moulds of up to 48 cavities to SHL Medical’s site in North Charleston. That kind of scale-up, done quickly and reliably, really shows the strength of our modular mould strategy in action. And with Superior Tooling being located in North Carolina, we’re able to provide more efficient support, ensuring minimal downtime and maximum productivity as production scales up.
Q Can you talk about the SMART platform? What makes it particularly suited to the needs of medical device assembly today?

Figure 3: The SMART assembly machine uses an array of robotic arms that are programmed to migrate device components from feeding systems to moving shuttles.
A The SMART assembly machine is another good example of our modular equipment strategy. The platform was developed to address a key gap in the market – the need for greater flexibility and quality in low- to medium-volume medical device assembly. Traditional manual or semi-automated systems are quicker to deploy but lack the consistency of full automation. On the other hand, fully automated systems offer high efficiency but typically require long lead times and higher investment, making them less practical for early-stage or high-mix projects.
With drug development accelerating and product pipelines growing, pharma companies need equipment that can adapt quickly without sacrificing quality. That’s where the SMART comes in. As a highly modular platform, it combines fully automated handling with standardised stations and interchangeable fixtures, allowing manufacturers to switch between device projects easily while maintaining the reliability and precision of automation (Figure 3). It’s an agile, scalable solution built for today’s fast-moving medical device landscape, and its value is already being recognised – we’ve been commissioned to build several new SMART lines set to be installed at sites in both the US and Europe.
“EARLY INVOLVEMENT ALSO ENABLES US TO TAKE A MORE INTEGRATED APPROACH FROM THE START, BRINGING TOGETHER EVERYTHING FROM INJECTION MOULDS AND SUB-ASSEMBLY EQUIPMENT TO TESTING AND FINAL ASSEMBLY SYSTEMS.”
Q How early in the process does SHL Advantec typically get involved with pharma or biotech partners, and what kind of impact does that early engagement have?
A Typically, and ideally, we’re involved right from the very beginning – at the first interaction between SHL Medical or the device developer and the pharma customer. Early involvement allows us to fully understand the project’s technical requirements and recommend the most appropriate path forward, whether that’s one of our flexible platforms or a tailored solution.
Early involvement also enables us to take a more integrated approach from the start, bringing together everything from injection moulds and sub-assembly equipment to testing and final assembly systems. This level of co-ordination is where SHL Advantec brings the most value – delivering fully integrated, turnkey solutions that streamline the entire development and manufacturing process.
Q The drug delivery device industry is experiencing significant shifts, including increased reliance on CDMOs, a push towards onshoring and regionalisation, and a heightened focus on supply chain resilience due to geopolitical pressures. How is SHL Advantec positioning itself in response to these evolving trends?
A These shifts – particularly the growing role of CDMOs and the push for regionalisation – are very much aligned with how we’ve built our offerings at SHL Advantec. To keep up with the fast-changing pharma landscape, CDMOs need highly flexible equipment that can support multiple device families and quickly adapt to changing project needs. Most of our final assembly and testing platforms were specifically designed with that in mind. They offer modularity, quick project changeovers and the ability to handle any scale of production requirements, from very early-stage pilot production to medium-volume output and extremely high-volume manufacturing, all designed without compromising quality (Figure 4).
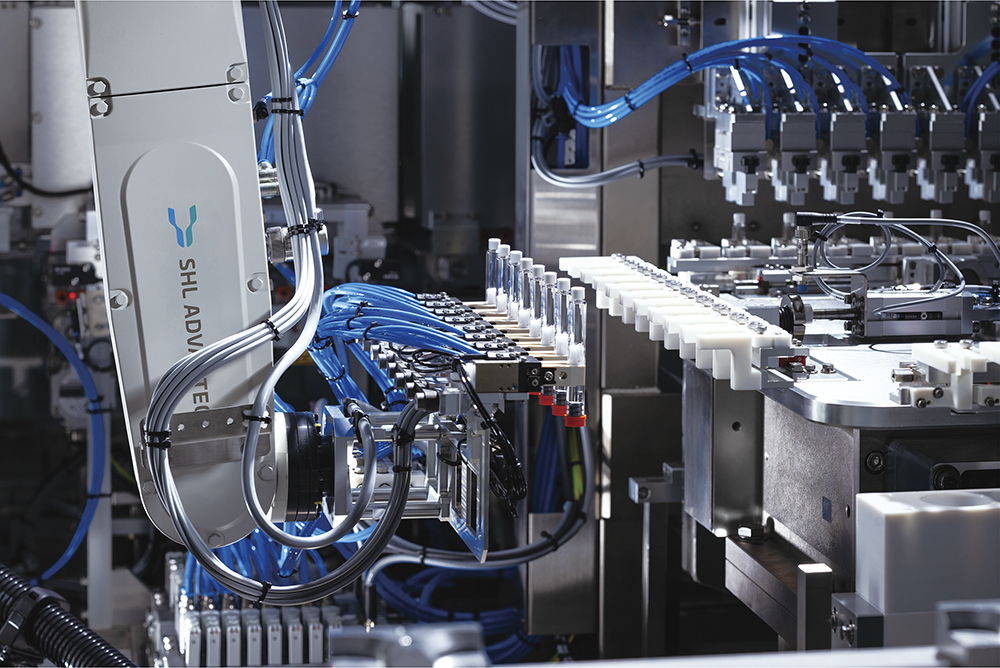
Figure 4: Fully modular final assembly machine with a fully automated primary container loader compatible with both tubs and trays.
Importantly, our equipment isn’t limited to devices developed by SHL Medical. Our platforms are designed to be flexible enough to accommodate device projects developed by other companies as well, making them well suited for CDMOs and pharma partners working across a diverse portfolio of drug delivery systems.
We also know that regional compliance is key to speed, which is why our platforms are built to meet varying regulatory and technical standards, such as the EU CE mark and US UL mark, so that they can be deployed efficiently across global manufacturing networks. Whether it’s moulding or assembly, our equipment helps customers – and their CDMO partners – move faster, stay flexible and meet market demands with more resilience.

