To Issue 176
Citation: Jost R, Käser D, “Divergent Design Philosophies in the Wearable Injector Segment”. ONdrugDelivery, Issue 176 (Sep 2025), pp 8–11.
Reto Jost and Daniel Käser detail how Ypsomed is approaching wearable injector design with YpsoDose and achieving large-volume requirements, consistent dosing and low residual waste, going on to explain how the company’s design choices are integrated with patient-centric features to ensure adherence and comfort.
As subcutaneous (SC) biologics increase in volume and complexity, the demand for wearable injectors enabling safe and effective at-home delivery continues to grow. Yet unlike the autoinjector and pen segments, which have largely converged on common standards, wearable injectors remain highly diverse in design. YpsoDose – a prefilled and ready-to-use large-volume wearable injector developed by Ypsomed – reflects a deliberate design philosophy that differentiates it within the fragmented design landscape of wearable injectors. YpsoDose prioritises consistent delivery, low residual volume and ease of use, helping to shape the large-volume wearable category.
IV LEAGUE DROPOUTS
Over the past decade, the biopharmaceutical industry has shifted towards higher-volume SC drug delivery. Driven by the rise of monoclonal antibodies, bispecifics, antibody-drug conjugates and other complex biologics, SC injection is becoming an increasingly preferred alternative to intravenous (IV) infusion for many indications. Compared with IV, SC administration offers faster dosing, greater flexibility in treatment location and improved patient experience. One widely cited example is daratumumab, which transitioned from a 3–7 hour IV infusion to a SC version deliverable in under five minutes, which now accounts for around 93% of usage in the US (Figure 1).

Figure 1: From IV to SC injection – YpsoDose enables the shift from hospital-based IV therapy to self-administered SC delivery.
This trend is made possible by advances in drug formulation, permeation enhancers and injection devices. As more therapies move into the home or outpatient setting, there is a growing need for delivery systems capable of handling volumes well beyond the 2 mL traditionally supported by autoinjectors. In a systematic review, Ypsomed found that over 100 large-volume SC candidates aim for doses of 5 mL or above. With more biologics entering the pipeline and patient care moving closer to home, the need for devices that support safe, convenient SC administration continues to grow.
“FOR DEVICE DEVELOPERS, THE CHALLENGE IS TO DESIGN LARGE-VOLUME INJECTION SYSTEMS THAT NOT ONLY MEET CLINICAL AND TECHNICAL REQUIREMENTS BUT ALSO FIT SEAMLESSLY INTO THE REALITIES OF SELF-CARE.”
This evolution concerns more than just dosing increase, it is a shift in how and where care happens. For device developers, the challenge is to design large-volume injection systems that not only meet clinical and technical requirements but also fit seamlessly into the realities of self-care, delivering efficiency, reliability and usability.
PATCH WORK: THE FRAGMENTED DESIGN LANDSCAPE OF WEARABLE INJECTORS
Pen injectors and autoinjectors have, over time, coalesced around a set of widely accepted design norms. Since the 1980s, cartridge-based pen injectors have evolved from prefilled reusable to prefilled multi-dose disposable, geared devices with easy-to-read displays, which are either manually or spring-driven. Single-use autoinjectors were first launched in 2006, and today they are usually based on two-step, push-on-skin mechanisms that are activated once the needle is fully inserted into the skin. The volumes delivered by single-use autoinjectors have increased to cover 1.0, 2.25 and most recently 5.5 mL prefilled syringes.
Traditionally, larger-volume SC injections have relied on reusable infusion pumps for delivering drugs in niche applications, such as apomorphine for treating Parkinson’s disease or gamma-globulins for immunomodulatory therapies. The emergence of new antibody therapies over the past 10–15 years has driven the need for larger-volume SC injections in the 5–20 mL range. In response, wearable injection devices have evolved alongside large-volume handheld autoinjectors to meet the requirements of these new treatment regimens.
It is the wearable nature of these systems, and the relatively recent emergence of the segment in comparison with pens and autoinjectors, that has led to considerable design divergence. While pens and autoinjectors tend to centre on faster injection times, simplified handheld actuation and integrated sterile drug paths (a cartridge with pen needle or a staked needle syringe), wearable injectors show a remarkable diversity in design. Differences span device shape, fluid path, patch technology, drive mechanisms, filling methods, user-assembly requirements and sterilisation strategies.
Against this backdrop, selecting the right design is more critical than ever. With so many design directions being followed in parallel by different design manufacturers, the implications for usability, reliability, cost-effectiveness and scalability are significant, making it even more critical for pharmaceutical companies to carefully consider their options when deciding on potential delivery devices.
Faced with this open design space, Ypsomed pursued a focused, pragmatic approach. Rather than following completely novel but unproven architectures, Ypsomed made foundational decisions that reflect real-world use cases and pharmaceutical constraints. The first such decision was to create a consistent injection experience for every dose.
FORCE MAJEURE: INJECTION CONSISTENCY AND EXTERNAL VARIABLES
While mechanical injection systems may appear simple and cost-efficient, they can introduce inconsistency into the drug delivery process. Spring-driven mechanical devices often exhibit declining force profiles as the spring decompresses, leading to fluctuating flow rates during the injection. In handheld autoinjectors we can already see variance in injection time, though the variance is negligible given the injection event lasts a matter of a few seconds. These limitations naturally become more pronounced with large-volume or high-viscosity formulations, where an injection may last up to 30 minutes.
YpsoDose avoids these pitfalls through its motor-driven push-on-plunger system. Unlike spring-based systems, the motor delivers consistent force throughout the injection event, independent of external conditions. This design enables a steady, controlled flow profile even under assumed worst-case conditions and reliable delivery of 10 mL at viscosities up to 50 cP in 10 minutes (Figure 2). The result is a system that delivers not just the correct volume but a consistent experience.
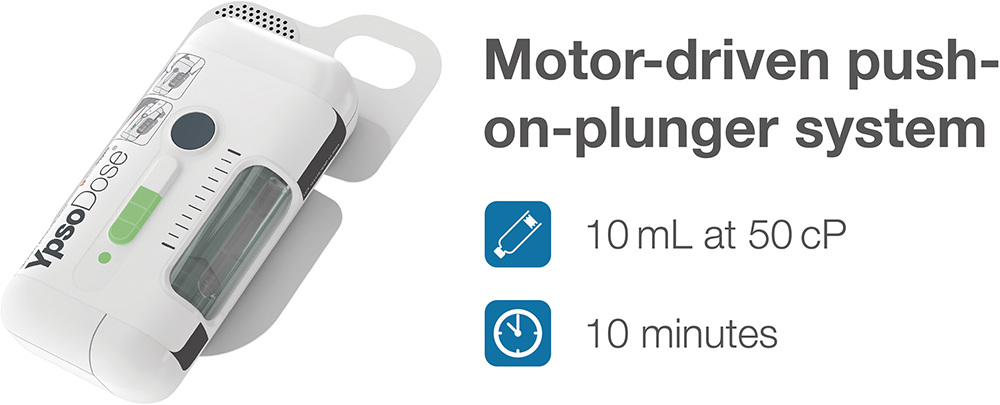
Figure 2: YpsoDose administers 10 mL at 50 cP in 10 minutes using a motor-driven push-on-plunger system – ensuring reproducibility even with challenging formulations.
External factors such as variations in temperature, orientation and injection site can also adversely affect delivery. As YpsoDose is motor-driven and sensor-supported, it performs reliably across a range of environmental and patient-specific variables. Skin-contact sensors verify correct placement before initiating the injection, reducing the risk of user error, and its motor drive allows for delivery across a wider range of temperatures. This stability translates into a smoother experience for patients and more dependable outcomes for healthcare providers. For treatments requiring long-term therapy adherence, this consistency supports trust and ongoing engagement. While this represents real value for patients, there is another key aspect that provides more direct value for pharma customers – minimising residual drug volume.
RESIDUAL VOLUME: THE SILENT DRAIN ON DRUG DELIVERY
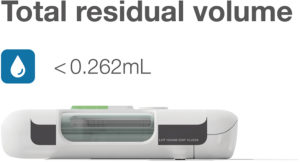
Figure 3: YpsoDose achieves a total residual volume for the system of <0.262 mL,
helping to reduce overfill, cut drug waste and protect valuable biologic formulations.
Two major factors contribute to drug loss in wearable injectors. Firstly, manual filling or transfer steps may introduce variability and loss; secondly, residual drug left in the device after injection due to fluid path design. These factors can lead to significant cumulative waste, particularly for high-cost biologics.
In contrast to, for example, suction-based delivery, YpsoDose was designed from the outset to minimise residual volume. YpsoDose integrates a prefilled cartridge with a simplified, optimised fluid path consisting of two cannulas and a short connecting tube. The result is a total residual volume for the system of <0.262 mL (<0.015 mL device fluid path, <0.247 mL cartridge) and zero waste from manual patient filling (Figure 3).
“BY MINIMISING DRUG WASTE, YPSODOSE REDUCES THE OVERFILL REQUIREMENT AND HELPS TO PRESERVE VALUABLE APIs.”
These numbers matter for pharma and this efficiency is not a secondary feature; it is integral to the device’s value. By minimising drug waste, YpsoDose reduces the overfill requirement and helps to preserve valuable APIs. Yet the implications extend beyond pharmacoeconomics. Reduced residual volume contributes to environmental sustainability by minimising chemical waste and reducing the carbon footprint associated with manufacturing and disposal. For pharmaceutical companies facing cost pressures and environmental, social and governance expectations, this design choice makes a compelling case.
MORE THAN JUST VOLUME AND FLOW
While minimal residual volume and consistent injection performance are foundational, they are only part of what makes YpsoDose a complete solution. Usability, safety and reliability are also central to the device’s value, each informed by human factors testing and industrial design decisions made to reduce user burden and improve confidence.
YpsoDose incorporates several other features designed to improve usability and confidence for both patients and pharmaceutical partners. An example of core patient centricity is the audio and visual feedback features, providing real-time cues that guide the user through the injection process, making it intuitive and reassuring. Likewise, electronic skin-contact sensors ensure that the injection is only initiated when proper contact with the skin is detected, reducing the risk of improper use and providing peace of mind. Flexibility is also built in, with the device supporting programmable injection speeds of up to 3.0 mL/min, allowing for the accommodation of different formulations and individual patient tolerances. Additionally, built-in sterility enables final assembly outside a cleanroom, reducing manufacturing and integration complexity for pharmaceutical companies.
“YPSODOSE OFFERS A SOLUTION THAT BALANCES INNOVATION WITH RELIABILITY, REVOLUTIONISING SELFCARE AND HELPING ARTNERS BRING THERAPIES TO MARKET WITH CONFIDENCE.”
Taken together, these design choices deliver not only a technically advanced device but one that is practical, scalable and aligned with the real-world demands of pharmaceutical development and patient use. YpsoDose offers a solution that balances innovation with reliability, revolutionising self care and helping partners to bring therapies to market with confidence.
Figure 4: YpsoDose is a complete, clinic- and market-ready solution, supported by expert partners in primary packaging (SCHOTT Pharma) and final assembly (ten23 health), ensuring end-to-end project confidence.
CONCLUSION
As the number of large-volume SC biologics increases, careful evaluation of device design is critical. YpsoDose addresses primary sources of delivery inefficiency – residual volume and injection inconsistency – with a platform built for reliability. Yet advanced features alone are not enough. YpsoDose is part of a broader solution ecosystem, supported by partnerships across the supply chain, including the primary container (SCHOTT Pharma), fill finish, and final assembly (ten23 health), ensuring a smooth, integrated path to clinic and market for customers (Figure 4). Clinically and commercially ready, YpsoDose enables easier adoption and faster market access for pharmaceutical partners.

