To Issue 176
Citation: Malica C, Gross C, “Symbioze® – the Electromechanical On-Body Drug Delivery System for Large-Volume Immunotherapies”. ONdrugDelivery, Issue 176 (Sep 2025), pp 48–52.
Corinne Malica and Cécile Gross discuss the challenges involved in developing a wearable device for on-body drug delivery of advanced biologics, with a focus on the immunotherapy sector, and the technical solutions that can overcome them, using Nemera’s Symbioze® as an example.
The increasing use of biologic therapies such as large-molecule drugs, especially monoclonal antibodies and other immunotherapies for cancer and autoimmune diseases, has led to new advanced drug delivery systems entering development. Historically, many of these biologics require intravenous (IV) infusions in clinics due to their high doses and volume requirements, placing a significant burden on patients and healthcare systems. Subcutaneous (SC) administration offers a patient-friendly alternative – reducing infusion times from hours to minutes and enabling treatment outside of hospitals. However, delivering the large volumes (>2 mL) and high viscosities necessary via the SC route has proven challenging with conventional drug delivery systems.
In response, on-body delivery systems (OBDSs) have emerged as a promising solution to enable the safe SC delivery of high-dose or higher-concentration biologics. These motorised wearable drug delivery systems attach to the patient’s body and autonomously infuse the medication at a controlled rate over an extended period, thereby facilitating at-home administration of complex cancer immunotherapies and other biologics that would otherwise require clinic-based infusions.
ENABLING SC DELIVERY OF LARGE-VOLUME BIOLOGICS
“IN THE FUTURE, THE INDUSTRY MAY SEE MORE IV ONCOLOGY DRUGS REFORMULATED FOR SC DELIVERY – USING OBDSs IF THE REQUIRED VOLUMES ARE VERY LARGE – TO OFFER PATIENTS THE FLEXIBILITY OF RECEIVING TREATMENT IN THE COMFORT
OF THEIR HOME OR LOCAL CLINIC.”
Conventional autoinjectors administer up to 2 mL of drug substance and rely on spring forces to deliver the drug in a rapid timeframe (10–15 seconds) – a method that can become impractical and painful for patients as volume or viscosity increases. OBDSs address these challenges by enabling the delivery of volumes of 20–30 mL over extended periods (from several minutes up to an hour) at controlled flow rates that can potentially mitigate pain and tissue stress. In the future, the industry may see more IV oncology drugs reformulated for SC delivery – using OBDSs if the required volumes are very large – to offer patients the flexibility of receiving treatment in the comfort of their home or local clinic.
SYMBIOZE® – THE STATE-OF-THE-ART ON-BODY DELIVERY SYSTEM PLATFORM
Nemera’s Symbioze® is an OBDS platform designed to accommodate the demanding requirements of biologic drug delivery. It incorporates several key trends in its design – a reusable/disposable hybrid architecture for sustainability, large-volume capacity and connectivity. The reusable subassembly contains the motor, battery and electronics, and can be recharged and used across multiple injections, with the user inserting a new disposable cartridge each time. This design prioritises sustainability, reducing the electronic waste generated per dose by having a lifelong core module with a two-year shelf life. The disposable unit includes a preloaded standard glass pharmaceutical cartridge, meaning that the patient does not manually handle the cartridge.
During injection, Symbioze® features a soft cannula, which is automatically inserted using a hidden needle, and is supplemented by connectivity via Bluetooth and near-field communication (NFC), enabling injection tracking and even drug identification by verifying the correct drug cartridge via an NFC tag. The user interface features a simple one-button activation approach and offers audio-visual feedback, along with an optional smartphone app for guidance. The combination of a modular reusable design with high-volume capability, typically from 20 to 30 mL, and digital integration positions Symbioze® as a flexible platform for a range of biologics and chronic conditions.
DESIGN CHALLENGES AND TECHNICAL SOLUTIONS
Injectability of High-Viscosity Drugs
Large biologic formulations often have high viscosities, making them difficult to push through a conventional thin needle. The Symbioze® OBDS tackles this with a powerful miniaturised motor that can sustain controlled flow rates for very viscous solutions, typically up to 50 cP. It also facilitates programmable delivery profiles to accommodate drug characteristics and minimise injection force (Figure 1).
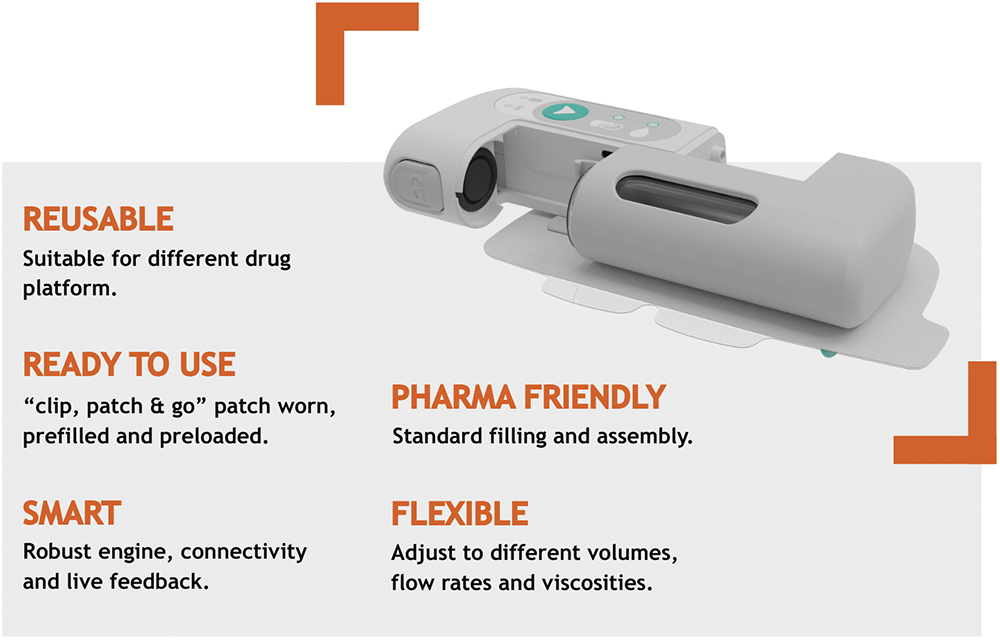
Figure 1: Symbioze® OBDS platform key features and benefits.
Patient Comfort and Tolerability
Drug delivery devices must be designed to avoid pain and fear as much as possible. OBDSs address this through slower infusion rates, thereby reducing the acute tissue distension that causes pain, and ergonomic cannula designs. To this end, Symbioze® was designed with the following features:
- Injection via a soft cannula to improve comfort during long injections
- The rigid needle for inserting the cannula is retracted at the start of injection, meaning that the patient never sees the needle and any risk of needlestick injury is avoided
- An adhesive patch that secures the device to the skin without any side effects
- Audible and visual feedback to reassure the user that the dose is being delivered correctly
- Large, tactile button and intuitive indicators that make the injection experience as seamless as possible for patients of all ages, including those with limited dexterity
- A combination of controlled flow, user-friendly and intuitive interface that maximises comfort and confidence during long SC injections.
“THE DEVICE IS ACTIVATED BY ONE BUTTON AND IS READY TO USE AS SOON AS THE DISPOSABLE UNIT IS ASSEMBLED, PREVENTING USER ASSEMBLY ERRORS AND MAKING TRAINING EASIER.”
Usability and Human Factors
Effective solutions include minimising user steps and providing clear instructions (Figure 2). For Symbioze®, extensive human factors studies were conducted to streamline the injection steps and optimise its intuitiveness (Figure 3). The device is activated by one button and is ready to use as soon as the disposable unit is assembled, preventing user assembly errors and making training easier. Furthermore, the Symbioze® platform incorporates multiple feedback mechanisms, including lights, an injection progress meter and audio cues at the start and completion of the injection.

Figure 3: Human factors studies by Insight by Nemera.

Figure 2: Clear instructions for use are crucial for therapy success.
Symbioze® is designed to ensure a safe connection between its disposable and reusable parts, guaranteeing that sterility is maintained throughout the injection (Figure 4). In addition, the device will not inject drug unless it is well positioned on the body, as well as assembled with the expected drug. These features guide users through the process and significantly lower the risk of misuse, which is crucial given that patients may be self-administering. Robust risk mitigation, including an automatic signal if an error is detected, is built-in to avoid any device malfunctions.
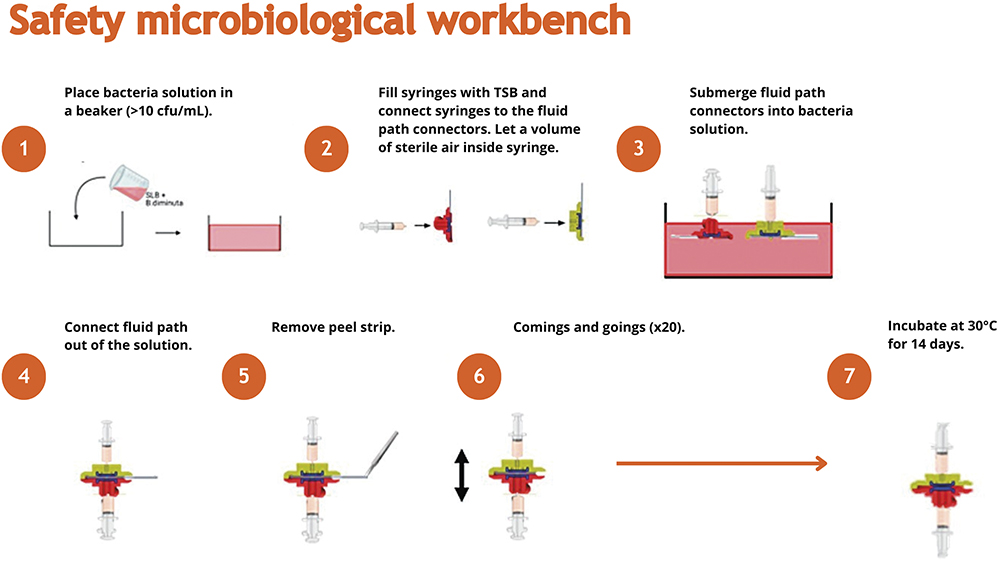
Figure 4: Testing methodology for ensuring the sterility of Symbioze®.
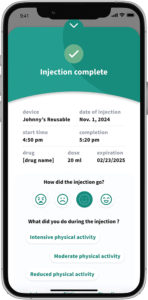
Figure 5: Symbioze® offers the possibility for connected accessory support.
Connectivity and Digital Health Integration
The Symbioze® platform increasingly offers connectivity features to support adherence and integrate with digital health ecosystems (Figure 5). This platform was conceived with connectivity in mind – it has built-in Bluetooth and can pair with a dedicated app or cloud system to manage injection schedules and monitor the device. The benefits of connectivity include reminders, training prompts and real-time feedback for patients, as well as enabling clinicians to remotely monitor dosing compliance.
OVERCOMING THE CHALLENGES
Taken together, the technical solutions discussed here enable Symbioze® to overcome the major hurdles of large-volume SC delivery. Through careful engineering, this OBDS ensures that even high-dose immunotherapies can be administered safely, comfortably and conveniently – meeting both the pharmaceutical requirements of biologic drugs and the practical needs of patients in the real-world use.
“SYMBIOZE® INTEGRATES SEVERAL CUTTING-EDGE FEATURES INTO ONE PLATFORM – LARGE-VOLUME CAPACITY, REUSABLE CORE FOR SUSTAINABILITY, SOFT CANNULA FOR COMFORT, PREFILLED DISPOSABLE UNIT FOR EASE OF USE AND FULL CONNECTIVITY FOR SMART THERAPY MANAGEMENT.”
In summary, Symbioze® integrates several cutting-edge features into one platform – large-volume capacity, reusable core for sustainability, soft cannula for comfort, prefilled disposable unit for ease of use and full connectivity for smart therapy management. Additionally, the modular, reusable design of Symbioze® reduces waste and enables the platform to be adapted across therapies. For pharmaceutical companies, Nemera’s Symbioze® platform can be customised according to the needs of a given drug’s profile (volume, viscosity, injection time) and market needs (target patient population and lifecycle management), whether it’s maximising sustainability with a reusable model or minimising user steps.
CONCLUSION
Motorised OBDSs represent an innovative approach for administering large-volume biologics, bridging the gap between potent new therapies and patient-centric care. By learning from past devices and addressing challenges in injectability, comfort, sterility and connectivity, Symbioze® has demonstrated how to offer a more flexible solution for home-based immunotherapy that could enhance quality of life for patients. This potential could enable the pharma industry to unlock new opportunities and improve patient outcomes in cancer care through the power of innovative drug delivery.

