To Issue 178
Citation: Zeiss B, Lee L, Cordier S, “More Than the Sum of its Parts: How Supplier Collaboration Drives Successful Combination Products”. ONdrugDelivery, Issue 178 (Oct 2025), pp 64–70.
Bernd Zeiss, Lucy Lee and Sébastien Cordier discuss the multitudinous advantages of collaboration between suppliers and pharma partners, from boosting innovation to optimising component choices for device development early on, all while ensuring compliance with global regulatory standards.
In the dynamic field of pharmaceutical combination products, synergy between component suppliers and between suppliers and pharmaceutical companies is essential. Collaboration is especially crucial in the development of prefilled syringes (PFSs) for integration into autoinjectors. Such relationships go beyond simple transactions; they are true partnerships that enhance product performance, ensure regulatory compliance and anticipate future needs. Successful partnerships ultimately result in patient-centric combination products that are greater than the sum of their parts.
With the introduction of new industry guidance, such as the US FDA’s Essential Drug Delivery Outputs (EDDO) for Devices Intended to Deliver Drugs and Biological Products,1 close collaboration between companies is more critical than ever for the successful development of combination products.
“THIS JOINT EFFORT EXEMPLIFIES HOW SHARED EXPERTISE AND EARLY-STAGE DATA GENERATION CAN STREAMLINE DEVELOPMENT AND SUPPORT PHARMA COMPANIES TO ACHIEVE OPTIMAL OUTCOMES FOR THEIR COMBINATION PRODUCTS.”
Given the essential role each component supplier plays in creating robust systems, the key question becomes: how can we reduce R&D burden for pharmaceutical partners, minimise the risk of product failure during development and, at the same time, accelerate time to market? In response to this question, Gerresheimer, Aptar Pharma and SHL Medical collaborated to collect functional and performance data on an autoinjector system (Figure 1). The system incorporated Gerresheimer’s premium quality Gx® Elite syringe and an Aptar Premium Coat® plunger stopper within the Molly® autoinjector platform from SHL Medical. This joint effort exemplifies how shared expertise and early-stage data generation can streamline development and support pharma companies to achieve optimal outcomes for their combination products.
ENSURING COMPLIANCE AND PERFORMANCE IN AUTOINJECTOR-BASED COMBINATION PRODUCTS
Incorporating a PFS into an autoinjector presents unique challenges, as a high level of precision, reliability and seamless integration of components is required. Each element, such as the syringe barrel, elastomer and autoinjector mechanism, must function together to ensure the overall safety and effectiveness of the final combination product. Component suppliers play a critical role by proactively testing and validating their products against international standards, including the new United States Pharmacopeia (USP) <382> and USP <1382> chapters, ISO 11040-8 for PFSs, ISO 11608-1 and 11608-5 for autoinjectors and the FDA’s EDDO.1
USP <382>
This general chapter addresses the fitness-for-use functional requirements for packaging/delivery systems that are intended for parenteral dosage forms and include primary packaging components partially or completely made of elastomeric material. With regards to PFSs, break-loose and gliding forces (BLGF), needle shield functionality, plunger seal integrity, as well as integrity testing, are all within the scope of USP <382>.
ISO 11040-8
ISO 11040-8 is part of the ISO 11040 series, which focuses on packaging systems and components for pharmaceutical use. ISO 11040-8 deals specifically with “finished PFSs” used to deliver medications. This norm covers design, functionality, safety and materials of PFSs to ensure that they meet the requirements necessary for safe and effective use without compromising safety or integrity. It includes aspects such as dimensional requirements, performance testing and usability features critical to healthcare professionals and end-users.
ISO 11608-1 and ISO 11608-5
The ISO 11608 series, especially Parts 1 and 5, addresses functional requirements and test methods for automated functions in needle-based injection systems. The standards outline the necessary criteria to evaluate devices that incorporate automated features such as dose delivery and needle insertion. Key elements covered in this norm include dose delivery mechanisms, performance testing, safety, usability and mechanical requirements. It helps manufacturers to design and develop reliable and safe devices for self-administering drugs, facilitating regulatory approval and enhancing user trust in automated medical devices.
FDA EDDO for Devices Intended to Deliver Drugs and Biological Products
When developing devices intended for the delivery of drugs and biological products it is, of course, critical to consider essential drug delivery outputs for efficacy, safety and regulatory compliance. The FDA provides guidance on these aspects. Suppliers can pre-test some EDDO aspects to reduce burden at later stages of combination product development. These include dose accuracy and precision, rate of delivery, user interface and usability, reliability and robustness, safety and risk mitigation.
LAYING THE FOUNDATION FOR SUCCESSFUL COMBINATION PRODUCT DEVELOPMENT THROUGH SYSTEM INTEGRATION
Based on their knowledge and experience of the system’s physical performance, component suppliers can generate valuable, drug-agnostic platform data by making informed assumptions about typical use cases. This approach enables performance verification of autoinjector systems and syringe components through key tests, including:
- Needle-shield Pull-off Force (PoF): Assessing ease of removal while maintaining sterility.
- BLGF: Simulating injection forces with model liquids.
- Autoinjector Fit: Ensuring dimensional and functional compatibility.
“BY PROVIDING SUCH COMPREHENSIVE DATA, SUPPLIERS HELP PHARMACEUTICAL COMPANIES SELECT OPTIMAL COMPONENTS WITHOUT STARTING DEVELOPMENT FROM SCRATCH, WHICH CAN ACCELERATE TIMELINES AND REDUCE RISK.”
By providing such comprehensive data, suppliers help pharmaceutical companies select optimal components without starting development from scratch, which can accelerate timelines and reduce risk. In addition to platform development, supplier-affiliated or independent laboratories can conduct stability studies, clinical simulations and human factors testing. Such a collaborative, forward-looking approach lays the foundation for successful combination product development and regulatory design verification.
Risk assessment also plays a crucial role in system testing. Suppliers must evaluate the risk of component failure and develop mitigation strategies. For instance, the predictability of BLGFs is generally high, and the spring force of the autoinjector can be adapted to meet specific performance requirements (Table 1).
| System performance |
Essential performance requirements |
Risk of failure – supplier assessment |
Contributing part of the system | Risk of failure |
Risk-mitigation strategies through supplier collaboration |
||
| Components | Syringe | Autoinjector | |||||
| Break-loose and gliding force | Forces enable manual injection or autoinjector integration | Mostly predictable based on solution properties | ✓ | ✓ | Low to moderate | Generate platform datasets to predict real case results. Spring force of autoinjector can be adapted for compatibility with gliding and break-loose forces | |
| Finger flange and cone break | PFS resistance higher than spring force | Drug independent | ✓ | ✓ | Low | Comparison of PFS and autoinjector specifications. Resistance tests |
|
| Needle shield and cap removal force | Cap gripping force higher than RNS pull-off | Drug independent | ✓ | ✓ | ✓ | Low | Determination of RNS pull-off force, and comparison with autoinjector specification |
| Dimensional fit into autoinjector |
Compatibility of key dimensions | Drug independent | ✓ | ✓ | Low | Comparison of key dimensional requirements and tolerances | |
| Administration time | Acceptable time for subcutaneous injection |
Mostly predictable based on solution properties | ✓ | ✓ | ✓ | Low to moderate | Integration of multiple parameters to anticipate results (BLGF, needle length, needle diameter, drug viscosity, etc.) |
Table 1: Example of risk assessments conducted by component suppliers for autoinjector-based combination products.
Interface of Syringe Barrel and Elastomer Components
The interfaces between the syringe barrel and elastomer caps/needle shields, as well as the elastomer plunger stopper, are critical for the device’s physical functionality. Figure 1 shows physical influencing factors, which can be either drug dependent or drug agnostic.
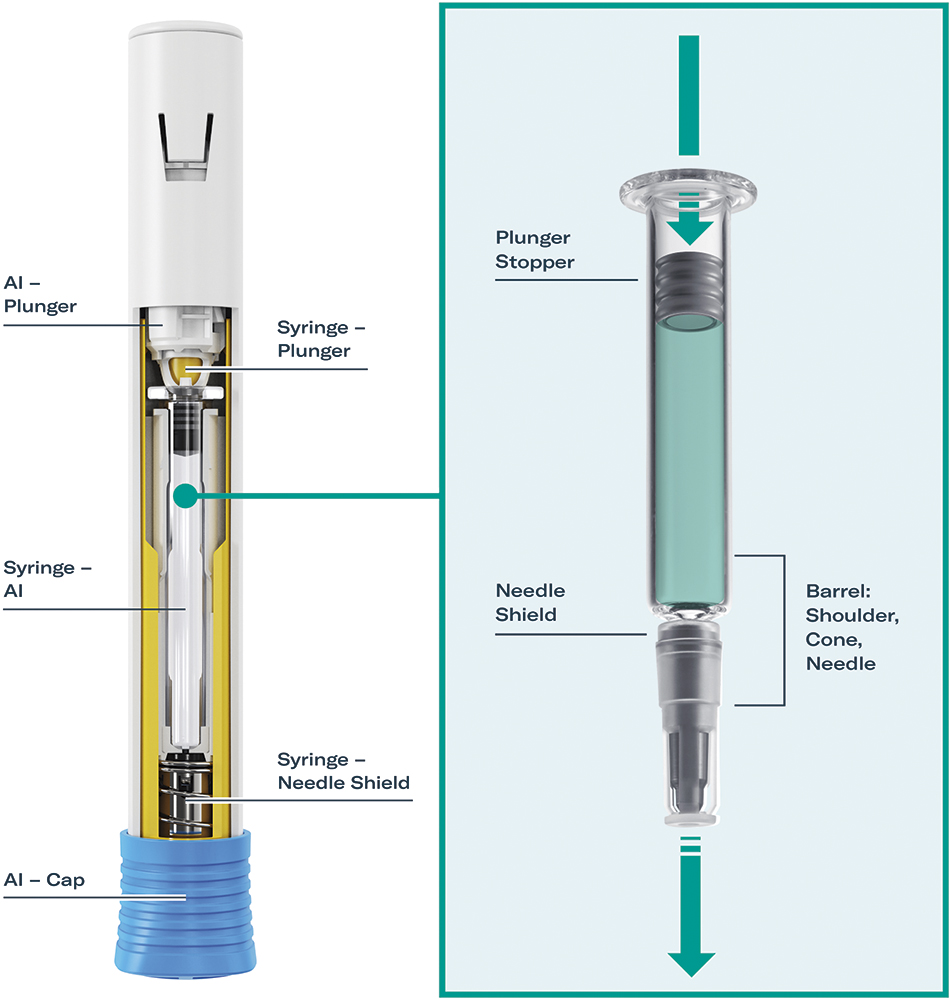
Figure 1: Interface between the autoinjector, syringe, needle shield and plunger stopper. The image shows SHL Medical’s Molly® modular platform autoinjector with Gerresheimer’s Gx® Elite 2.25 mL long glass syringe and Aptar Pharma’s PremiumCoat® plunger stopper.
USP <382> and USP <1382> chapters address and give guidance for the functionality data of these components. As a pharma company brings the final drug product to the market, it is ultimately their responsibility to comply with these standards. However, component suppliers can pre-test important drug-agnostic functional aspects of the device. These tests can include bracketing assessments with syringes filled with water for injection, or other model liquids to establish baseline performance data. Bracketing in this context means that well-functioning combinations will be suggested to pharma partners as a platform, while less optimal systems will not be recommended in the first place. Testing and evaluating various syringe barrel-to-elastomer interfaces help define the container, which eventually gets assembled inside the autoinjector. Such platform test data are invaluable for defining the best primary packaging combination mounted inside the device according to its intended use. For example, BLGF curves from test labs, like those at Gerresheimer and Aptar Pharma, help in selecting the optimal components.
Drug-Agnostic Versus Drug-Dependent Syringe Features
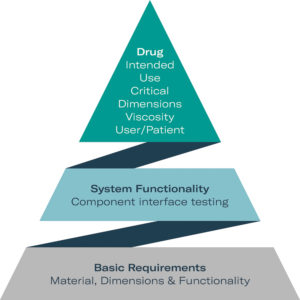
Figure 2: Three levels of syringe and device functionality: alongside basic material information, suppliers can contribute to general functionality by providing platform test data prior to testing the finished drug product.
Reliable testing of basic requirements can be carried out by component suppliers if they work collaboratively. Some tests are entirely independent from the drug properties and mainly material-related. They can easily be carried out by suppliers alone. System requirement tests depend on the properties of the liquid but are mainly physical by nature. Hence, tests can be carried out with model liquids so the device as a system can be adapted by suppliers prior to drug substance exposure. Data from supplier co-operation help to identify the optimal syringe system and autoinjector layout. When it comes to the finished drug product of a PFS or a syringe mounted into an autoinjector, true drug-dependent testing needs to be performed, which can only be undertaken in pharma labs (Figure 2).
The rigid needle shield (RNS) PoF is drug-agnostic by nature, which depends on:
- Dimensions of the cone
- Needle outer diameter (OD)
- RNS design and material
- Siliconisation
- Sterilisation influence
- Storage time and conditions.
A major liquid-dependent syringe property is combined BLGF. Mainly, the gliding force is strongly influenced by:
- Barrel material
- Length and inner diameter (ID)
- Needle length and ID
- Drug viscosity
- Elastomer type and design
- Siliconisation type and amount
- Stoppering method
- Storage time and conditions
- Speed parameters applied.
To assess gliding force without a defined drug product, syringe suppliers can generate orientating data with model liquids that simulate drug properties.
Gerresheimer and Aptar Pharma conducted a series of tests (Figure 3) of the 2.25 mL Gerresheimer Gx® Elite syringe to show general performance of the syringe system. Often, autoinjectors are stored at lower temperatures, for example, in a patient’s fridge before use. Thus removing the cap can lead to unacceptably high PoF. Tests show that storage at 5°C has no significant influence on PoF after adapting the cone-elastomer interface. RNS PoF with optimised cone/RNS interface was tested at room temperature with a mean of 10.59 N and in cold storage at 5°C with a mean of 12.16 N. These were considered to be good results, as standard PoF values for PFSs today are 30 N or higher.
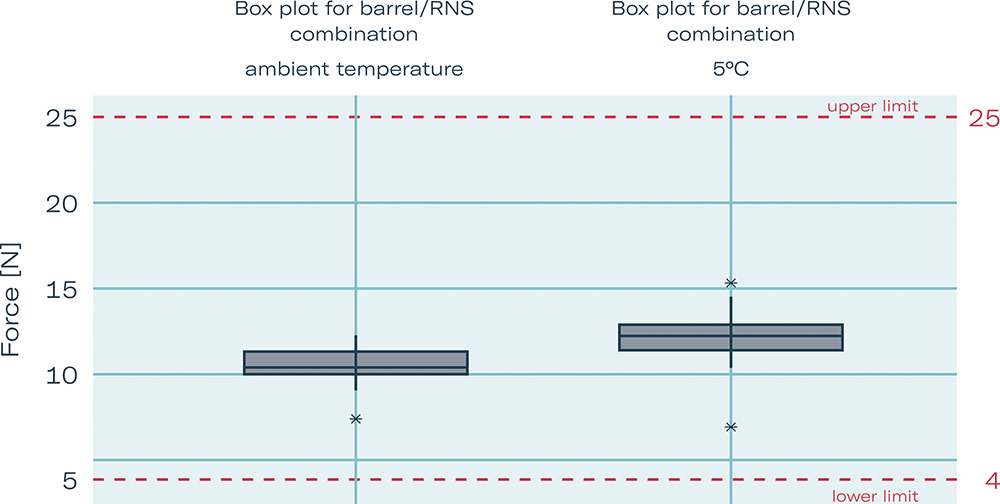
Figure 3: Box plot showing RNS pull-off force with optimised cone/RNS interface, ambient temperature versus cold storage 5°C (worst case).
Interface of PFS and Autoinjector
Achieving seamless integration between a syringe barrel and autoinjector is essential, as it directly impacts the functionality, safety and reliability of the final combination product. Some performance parameters are drug-agnostic and can be pre-assessed early in development. These include factors such as needle-shield removal force, activation force and overall mechanical compatibility between components. More functional parameters such as BLGF, or injection time are dependent on the physical properties of the drug formulation. They can also be pre-assessed by suppliers with information provided by pharma partners.
“PRE-ASSESSMENTS ALLOW SUPPLIERS TO IDENTIFY POTENTIAL RISKS, OPTIMISE COMPONENT FIT AND ENSURE COMPLIANCE WITH RELEVANT STANDARDS BEFORE DRUG-SPECIFIC TESTING BEGINS.”
Such pre-assessments allow suppliers to identify potential risks, optimise component fit and ensure compliance with relevant standards before drug-specific testing begins (Figure 2). By using platform performance data, suppliers can recommend the most suitable materials and components for the combination product. These shared, early-stage data not only streamline development but also de-risk the pharmaceutical company’s choice of primary container, reducing costly design iterations and accelerating time to market.
Drug-Agnostic and Liquid-Dependent System Features
There are basic liquid-agnostic features of PFSs in autoinjectors that need to be tested and approved, such as dimensional fit, needle-shield removal force, activation force (choosing fitting spring) and penetration force (needle OD and gauge). More complex are liquid-dependent syringe-autoinjector system features, such as dose accuracy, delivered dose, average glide force and injection time. Needle ID, drug viscosity, drug density and temperature, as well as needle extension, can heavily affect the system’s functionality.
The liquid-dependent performance features of average glide force and injection time were tested by Gerresheimer, SHL Medical and Aptar Pharma in a joint study. As with the PoF tests shown in Figure 3, a 2.25 mL Gx® Elite syringe with 27 G thin wall and 0.28 mm ID was used for testing. The syringe was filled with a glycerin solution of 15 cP, stoppered with the PremiumCoat® plunger from Aptar Pharma and assembled into the Molly® autoinjector from SHL Medical. Average glide force was 3.66 N at 0.5 mm/s and 33.01 N at a higher injection speed of 5 mm/s. With this system layout, injection time was measured at 9.41 s, which is a frequently used target value for autoinjector applications (Figure 4).
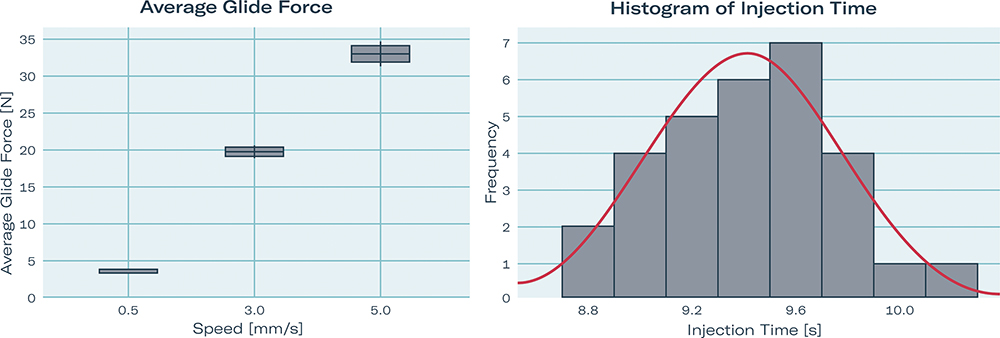
Figure 4: Liquid-dependent performance features: average glide force (1) and injection time (2) of a 2.25 mL Gx® Elite syringe 27 G thin wall, ID 0.28 mm, with PremiumCoat® plunger mounted in SHL Molly; tested with 15 cPs water-based solution.
“THE MORE LIQUID-RELATED INFORMATION AVAILABLE EARLY ON, THE EASIER IT IS TO RECOMMEND AN AUTOINJECTOR SYSTEM.”
CONCLUSION
SHL Medical, Aptar Pharma and Gerresheimer demonstrate the critical role of collaboration between pharmaceutical companies and component suppliers in the successful development of autoinjector-based combination products. The more liquid-related information available early on, the easier it is to recommend an autoinjector system. From syringe barrels and elastomeric components to the autoinjector itself, each element must be precisely engineered and seamlessly integrated to meet the stringent performance and regulatory requirements of today’s combination drug delivery systems.
Close co-operation between suppliers and pharmaceutical partners enables early identification of liquid-agnostic performance factors. It also supports comprehensive risk assessments and provides access to robust platform data. This not only helps to de-risk the selection of components – such as plunger stoppers, needle shields and primary containers – but can also accelerate development timelines and reduce the need for costly, late-stage redesigns.
By applying their respective areas of expertise, suppliers can recommend optimal materials and configurations tailored to pharmaceutical needs. Pharma companies benefit from data-driven decisions and more efficient progression through development and regulatory milestones. Ultimately, the final combination products are not only safe and effective, but also patient centric and commercially viable.
In short, seamless collaboration between device, component and pharmaceutical stakeholders establishes a foundation for innovation, efficiency and long-term success – delivering solutions that are truly greater than the sum of their parts.
REFERENCE
1. “Guidance for Industry: Drug Delivery Outputs for Devices Intended to Deliver Drugs and Biological Products”. US FDA, Jun 2024

